Biosynthesis of plant-specific stilbene polyketides in metabolically engineered Escherichia coli
- PMID: 16551366
- PMCID: PMC1435877
- DOI: 10.1186/1472-6750-6-22
Biosynthesis of plant-specific stilbene polyketides in metabolically engineered Escherichia coli
Abstract
Background: Phenylpropanoids are the precursors to a range of important plant metabolites such as the cell wall constituent lignin and the secondary metabolites belonging to the flavonoid/stilbene class of compounds. The latter class of plant natural products has been shown to function in a wide range of biological activities. During the last few years an increasing number of health benefits have been associated with these compounds. In particular, they demonstrate potent antioxidant activity and the ability to selectively inhibit certain tyrosine kinases. Biosynthesis of many medicinally important plant secondary metabolites, including stilbenes, is frequently not very well understood and under tight spatial and temporal control, limiting their availability from plant sources. As an alternative, we sought to develop an approach for the biosynthesis of diverse stilbenes by engineered recombinant microbial cells.
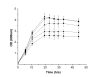
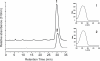
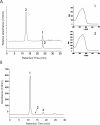
Results: A pathway for stilbene biosynthesis was constructed in Escherichia coli with 4-coumaroyl CoA ligase 1 4CL1) from Arabidopsis thaliana and stilbene synthase (STS) cloned from Arachis hypogaea. E. coli cultures expressing these enzymes together converted the phenylpropionic acid precursor 4-coumaric acid, added to the growth medium, to the stilbene resveratrol (>100 mg/L). Caffeic acid, added in the same way, resulted in the production of the expected dihydroxylated stilbene, piceatannol (>10 mg/L). Ferulic acid, however, was not converted to the expected stilbene product, isorhapontigenin. Substitution of 4CL1 with a homologous enzyme, 4CL4, with a preference for ferulic acid over 4-coumaric acid, had no effect on the conversion of ferulic acid. Accumulation of tri- and tetraketide lactones from ferulic acid, regardless of the CoA-ligase expressed in E. coli, suggests that STS cannot properly accommodate and fold the tetraketide intermediate to the corresponding stilbene structure.
Conclusion: Phenylpropionic acids, such as 4-coumaric acid and caffeic acid, can be efficiently converted to stilbene compounds by recombinant E. coli cells expressing plant biosynthetic genes. Optimization of precursor conversion and cyclization of the bulky ferulic acid precursor by host metabolic engineering and protein engineering may afford the synthesis of even more structurally diverse stilbene compounds.
Figures



Similar articles
-
Biosynthesis of plant-specific phenylpropanoids by construction of an artificial biosynthetic pathway in Escherichia coli.J Ind Microbiol Biotechnol. 2011 Oct;38(10):1657-65. doi: 10.1007/s10295-011-0954-3. Epub 2011 Mar 20. J Ind Microbiol Biotechnol. 2011. PMID: 21424580
-
Production of resveratrol from p-coumaric acid in recombinant Saccharomyces cerevisiae expressing 4-coumarate:coenzyme A ligase and stilbene synthase genes.Enzyme Microb Technol. 2011 Jan 5;48(1):48-53. doi: 10.1016/j.enzmictec.2010.09.004. Epub 2010 Sep 15. Enzyme Microb Technol. 2011. PMID: 22112770
-
Construction, expression, and characterization of Arabidopsis thaliana 4CL and Arachis hypogaea RS fusion gene 4CL::RS in Escherichia coli.World J Microbiol Biotechnol. 2015 Sep;31(9):1379-85. doi: 10.1007/s11274-015-1889-z. Epub 2015 Jun 20. World J Microbiol Biotechnol. 2015. PMID: 26092168
-
Combinatorial biosynthesis of non-bacterial and unnatural flavonoids, stilbenoids and curcuminoids by microorganisms.J Antibiot (Tokyo). 2008 Dec;61(12):709-28. doi: 10.1038/ja.2008.85. J Antibiot (Tokyo). 2008. PMID: 19194030 Review.
-
Biosynthesis of resveratrol and piceatannol in engineered microbial strains: achievements and perspectives.Appl Microbiol Biotechnol. 2019 Apr;103(7):2959-2972. doi: 10.1007/s00253-019-09672-8. Epub 2019 Feb 23. Appl Microbiol Biotechnol. 2019. PMID: 30798357 Review.
Cited by
-
Metabolic engineering of yeast and plants for the production of the biologically active hydroxystilbene, resveratrol.J Biomed Biotechnol. 2012;2012:579089. doi: 10.1155/2012/579089. Epub 2012 May 13. J Biomed Biotechnol. 2012. PMID: 22654481 Free PMC article. Review.
-
Saccharomyces Cerevisiae-An Interesting Producer of Bioactive Plant Polyphenolic Metabolites.Int J Mol Sci. 2020 Oct 5;21(19):7343. doi: 10.3390/ijms21197343. Int J Mol Sci. 2020. PMID: 33027901 Free PMC article. Review.
-
Natural Sources and Pharmacological Properties of Pinosylvin.Plants (Basel). 2022 Jun 9;11(12):1541. doi: 10.3390/plants11121541. Plants (Basel). 2022. PMID: 35736692 Free PMC article. Review.
-
Impact of heterologous expression of Cannabis sativa tetraketide synthase on Phaeodactylum tricornutum metabolic profile.Biotechnol Biofuels Bioprod. 2025 Apr 4;18(1):42. doi: 10.1186/s13068-025-02638-1. Biotechnol Biofuels Bioprod. 2025. PMID: 40186218 Free PMC article.
-
Reshaping the 2-Pyrone Synthase Active Site for Chemoselective Biosynthesis of Polyketides.Angew Chem Int Ed Engl. 2023 Jan 26;62(5):e202212440. doi: 10.1002/anie.202212440. Epub 2022 Dec 20. Angew Chem Int Ed Engl. 2023. PMID: 36398563 Free PMC article.
References
-
- Aoki T, Akashi T, Ayabe S. Flavonoids of leguminous plants: Structure, biological activity, and biosynthesis. J Plant Res. 2000;113:475–488. doi: 10.1007/PL00013958. - DOI
-
- Wolter F, Stein J. Biological activities of resveratrol and its analogs. Drugs Future. 2002;27:949–959. doi: 10.1358/dof.2002.027.10.856987. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

