MR imaging, MR spectroscopy, and diffusion tensor imaging of sequential studies in neonates with encephalopathy
- PMID: 16551990
- PMCID: PMC7976955
MR imaging, MR spectroscopy, and diffusion tensor imaging of sequential studies in neonates with encephalopathy
Abstract
Background: Although the imaging, spectroscopic, and diffusion characteristics of brains of infants with neonatal encephalopathy have been described, the time course during which these changes evolve is not clear. The results of sequential MR imaging studies--including anatomic MR imaging, proton MR spectroscopy, and diffusion tensor imaging (DTI)--of 10 patients enrolled prospectively in a study of neonatal encephalopathy are reported to help to clarify the time course of changes in different brain regions during the first 2 weeks of life.
Methods: Ten neonates were prospectively enrolled in a study of the evolution of MR findings in neonatal encephalopathy and were studied 2 (8 patients) or 3 (2 patients) times within the first 2 weeks of life. The MR examination included spin-echo T1 and T2-weighted images, DTI, and long echo time (288 milliseconds) proton MR spectroscopy. Diffusion parameters (diffusivity [D(av)], fractional anisotropy [FA], and individual eigenvalues) were calculated for 10 1-cm2 regions of interest in each hemisphere that were placed based on anatomic landmarks. D(av) and FA were then measured manually in the same areas on a workstation. Metabolite ratios (NAA/Ch, Cr/Ch, Cr/NAA, Lac/Ch, and Lac/NAA) were calculated in 7 regions of interest. Imaging appearance, diffusion parameters, and metabolite ratios were then evaluated longitudinally (comparing with other studies on the same patient at different times) and cross-sectionally (comparing all studies performed on the same postnatal day).
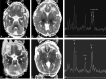
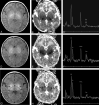
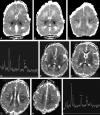
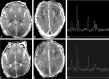
Results: In most of the patients a characteristic evolution of DTI and MR spectroscopy parameters was seen during the first 2 weeks after birth. Although the anatomic images were normal or nearly normal on the first 2 days after birth in most patients, abnormalities were detected on DTI (both visually and by quantitative interrogation of D(av) maps) and proton MR spectroscopy (abnormal metabolite ratios). These parameters tended to worsen until about day 5 and then normalize, though in several patients abnormal metabolite ratios persisted. Of interest, as areas of abnormal diffusivity pseudonormalized within one region of the brain they would develop in other areas. Therefore, the pattern of injury looked very different when imaging was performed at different times during this evolution.
Conclusion: Patterns of injury detected by standard anatomic imaging sequences, DTI sequences, and proton MR spectroscopy varied considerably during the first 2 weeks after injury. The appearance of new areas of reduced diffusion simultaneous with the pseudonormalization of areas that had reduced diffusion at earlier times can result in an entirely different pattern of injury on diffusivity maps acquired at different time points. Awareness of these evolving patterns is essential if studies are performed and interpreted during this critical period of time.
Figures



References
-
- Groenendaal F, Veenhoven EH, van der Grond J, et al. Cerebral lactate and N-acetyl-aspartate/choline ratios in asphyxiated full-term neonates demonstrated in-vivo using proton magnetic resonance spectroscopy. Pediatr Res 1994;35:148–51 - PubMed
-
- Preden CJ, Rutherford MA, Sargentoni J, et al. Proton spectroscopy of the neonatal brain following hypoxic-ischemic injury. Dev Med Child Neurol 1993;35:502–10 - PubMed
-
- Bryant DJ, Sargentoni J, Cox IJ, et al. Proton magnetic resonance spectroscopy of term infants with hypoxic ischaemic injury. In: Proceedings of the Society of magnetic resonance. San Francisco;1994;336
-
- Hanrahan JD, Sargentoni J, Azzopardi D, et al. Cerebral metabolism within 18 hours of birth asphyxia: a proton magnetic resonance spectroscopy study. Pediatr Res 1996;39:584–90 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
