Contribution of hemagglutinin/protease and motility to the pathogenesis of El Tor biotype cholera
- PMID: 16552036
- PMCID: PMC1418906
- DOI: 10.1128/IAI.74.4.2072-2079.2006
Contribution of hemagglutinin/protease and motility to the pathogenesis of El Tor biotype cholera
Abstract
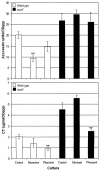
Vibrio cholerae is a highly motile organism that secretes a Zn-dependent metalloprotease, hemagglutinin/protease (HapA). HapA has been shown to have mucinase activity and contribute to the reactogenicity of live vaccine candidates, but its role in cholera pathogenesis is not yet clear. The contribution of motility to pathogenesis is not fully understood, since conflicting results have been obtained with different strains, mutants, and animal models. The objective of this work was to determine the contribution of HapA and motility to the pathogenesis of El Tor biotype cholera. To this end we constructed isogenic motility (motY) and mucinase (hapA) single and double mutants of an El Tor biotype V. cholerae strain. Mutants were characterized for the expression of major virulence factors in vitro and in vivo. The motility mutant showed a remarkable increase in cholera toxin (CT), toxin coregulated pilus major subunit (TcpA), and HapA production in vitro. Increased TcpA and CT production could be explained by increased transcription of tcpA, ctxA, and toxT. No effect was detected on the transcription of hapA in the motility mutant. The sodium ionophore monensin diminished production of HapA in the parent but not in the motility mutant. Phenamil, a specific inhibitor of the flagellar motor, diminished CT production in the wild-type and motY strains. The hapA mutant showed increased binding to mucin. In contrast, the motY mutation diminished adherence to biotic and abiotic surfaces including mucin. Lack of HapA did not affect colonization in the suckling mouse model. The motility and mucinase defects did not prevent induction of ctxA and tcpA in the mouse intestine as measured by recombinase-based in vivo expression technology. Analysis of mutants in the rabbit ileal loop model showed that both V. cholerae motility and HapA were necessary for full expression of enterotoxicity.
Figures




References
-
- Attridge, S. R., and D. Rowley. 1983. The role of the flagellum in the adherence of Vibrio cholerae. J. Infect. Dis. 147:864-872. - PubMed
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1995. Short protocols in molecular biology. John Wiley & Sons Inc., New York, N.Y.
-
- Benitez, J. A., L. Garcia, A. J. Silva, H. Garcia, R. Fando, B. Cedre, A. Perez, J. Campos, B. L. Rodriguez, J. L. Perez, T. Valmaseda, O. Perez, A. Perez, M. Ramirez, T. Ledon, M. Diaz, M. Lastre, L. Bravo, and G. Sierra. 1999. Preliminary assessment of the safety and immunogenicity of a new CTXΦ-negative hemagglutinin/protease-defective El Tor strain as a cholera vaccine candidate. Infect. Immun. 67:539-545. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

