A novel Staphylococcus aureus vaccine: iron surface determinant B induces rapid antibody responses in rhesus macaques and specific increased survival in a murine S. aureus sepsis model
- PMID: 16552052
- PMCID: PMC1418914
- DOI: 10.1128/IAI.74.4.2215-2223.2006
A novel Staphylococcus aureus vaccine: iron surface determinant B induces rapid antibody responses in rhesus macaques and specific increased survival in a murine S. aureus sepsis model
Abstract
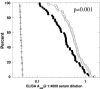
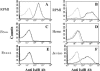
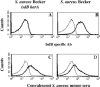
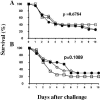
Staphylococcus aureus is a major cause of nosocomial infections worldwide, and the rate of resistance to clinically relevant antibiotics, such as methicillin, is increasing; furthermore, there has been an increase in the number of methicillin-resistant S. aureus community-acquired infections. Effective treatment and prevention strategies are urgently needed. We investigated the potential of the S. aureus surface protein iron surface determinant B (IsdB) as a prophylactic vaccine against S. aureus infection. IsdB is an iron-sequestering protein that is conserved in diverse S. aureus clinical isolates, both methicillin resistant and methicillin sensitive, and it is expressed on the surface of all isolates tested. The vaccine was highly immunogenic in mice when it was formulated with amorphous aluminum hydroxyphosphate sulfate adjuvant, and the resulting antibody responses were associated with reproducible and significant protection in animal models of infection. The specificity of the protective immune responses in mice was demonstrated by using an S. aureus strain deficient for IsdB and HarA, a protein with a high level of identity to IsdB. We also demonstrated that IsdB is highly immunogenic in rhesus macaques, inducing a more-than-fivefold increase in antibody titers after a single immunization. Based on the data presented here, IsdB has excellent prospects for use as a vaccine against S. aureus disease in humans.
Figures


References
-
- Anderegg, T. R., H. S. Sader, T. R. Fritsche, J. E. Ross, and R. N. Jones. 2005. Trends in linezolid susceptibility patterns: report from the 2002-2003 worldwide Zyvox Annual Appraisal of Potency and Spectrum (ZAAPS) Program. Int. J. Antimicrob. Agents 26:13-21. - PubMed
-
- Briles, D. E., C. Forman, S. Hudak, and J. L. Claflin. 1984. The effects of subclass on the ability of anti-phosphocholine antibodies to protect mice from fatal infection with Streptococcus pneumoniae. J. Mol. Cell. Immunol. 1:305-309. - PubMed
-
- Chu, R. S., T. McCool, N. S. Greenspan, J. R. Schreiber, and C. V. Harding. 2000. CpG oligodeoxynucleotides act as adjuvants for pneumococcal polysaccharide-protein conjugate vaccines and enhance antipolysaccharide immunoglobulin G2a (IgG2a) and IgG3 antibodies. Infect. Immun. 68:1450-1456. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

