Immunization with the Haemophilus ducreyi hemoglobin receptor HgbA protects against infection in the swine model of chancroid
- PMID: 16552053
- PMCID: PMC1418891
- DOI: 10.1128/IAI.74.4.2224-2232.2006
Immunization with the Haemophilus ducreyi hemoglobin receptor HgbA protects against infection in the swine model of chancroid
Abstract
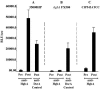
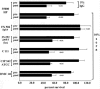
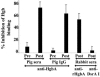
The etiologic agent of chancroid is Haemophilus ducreyi. To fulfill its obligate requirement for heme, H. ducreyi uses two TonB-dependent receptors: the hemoglobin receptor (HgbA) and a receptor for free heme (TdhA). Expression of HgbA is necessary for H. ducreyi to survive and initiate disease in a human model of chancroid. In this study, we used a swine model of H. ducreyi infection to demonstrate that an experimental HgbA vaccine efficiently prevents chancroid, as determined by several parameters. Histological sections of immunized animals lacked typical microscopic features of chancroid. All inoculated sites from mock-immunized pigs yielded viable H. ducreyi cells, whereas no viable H. ducreyi cells were recovered from inoculated sites of HgbA-immunized pigs. Antibodies from sera of HgbA-immunized animals bound to and initiated antibody-dependent bactericidal activity against homologous H. ducreyi strain 35000HP and heterologous strain CIP542 ATCC; however, an isogenic hgbA mutant of 35000HP was not killed, proving specificity. Anti-HgbA immunoglobulin G blocked hemoglobin binding to the HgbA receptor, suggesting a novel mechanism of protection through the limitation of heme/iron acquisition by H. ducreyi. Such a vaccine strategy might be applied to other bacterial pathogens with strict heme/iron requirements. Taken together, these data suggest continuing the development of an HgbA subunit vaccine to prevent chancroid.
Figures


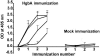
References
-
- Al-Tawfiq, J. A., K. R. Fortney, B. P. Katz, A. F. Hood, C. Elkins, and S. M. Spinola. 2000. An isogenic hemoglobin receptor-deficient mutant of Haemophilus ducreyi is attenuated in the human model of experimental infection. J. Infect. Dis. 181:1049-1054. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

