Pharmacokinetic study of paclitaxel in malignant ascites from advanced gastric cancer patients
- PMID: 16552811
- PMCID: PMC4124320
- DOI: 10.3748/wjg.v12.i9.1412
Pharmacokinetic study of paclitaxel in malignant ascites from advanced gastric cancer patients
Abstract
Aim: To examine the paclitaxel concentrations in plasma and ascites after its intravenous administration in patients with ascites due to peritonitis carcinomatosa resulting from advanced gastric cancer.
Methods: Two patients with ascites due to peritonitis carcinomatosa resulting from gastric cancer were included in this study. The paclitaxel concentrations in plasma and ascites were investigated for 72 h in case 1 and 168 h in case 2 after intravenous administration.
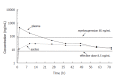
Results: The paclitaxel concentration in plasma peaked immediately after administration, followed by rapid decrease below the threshold value of 0.1 micromol (85 ng/mL) within 24 h. In contrast,the paclitaxel concentration in ascites increased gradually for 24 h after administration to a level consistent with the level found in plasma. After 24 h the level of paclitaxel in ascites and plasma became similar, with the optimal level being maintained up to 72 h following administration.
Conclusion: The concentration of paclitaxel in ascites is maintained within the optimal level for the treatment of cancer cells for up to 72 h after intravenous administration. Paclitaxel is a promising drug for the treatment of malignant ascites of gastric cancer.
Figures

References
-
- Rowinsky EK, Donehower RC. Paclitaxel (taxol) N Engl J Med. 1995;332:1004–1014. - PubMed
-
- Chang YF, Li LL, Wu CW, Liu TY, Lui WY, P'eng FK, Chi CW. Paclitaxel-induced apoptosis in human gastric carcinoma cell lines. Cancer. 1996;77:14–18. - PubMed
-
- Kim YH, Shin SW, Kim BS, Kim JH, Kim JG, Mok YJ, Kim CS, Rhyu HS, Hyun JH, Kim JS. Paclitaxel, 5-fluorouracil, and cisplatin combination chemotherapy for the treatment of advanced gastric carcinoma. Cancer. 1999;85:295–301. - PubMed
-
- Murad AM, Petroianu A, Guimaraes RC, Aragao BC, Cabral LO, Scalabrini-Neto AO. Phase II trial of the combination of paclitaxel and 5-fluorouracil in the treatment of advanced gastric cancer: a novel, safe, and effective regimen. Am J Clin Oncol. 1999;22:580–586. - PubMed
-
- Lokich JJ, Sonneborn H, Anderson NR, Bern MM, Coco FV, Dow E, Oliynyk P. Combined paclitaxel, cisplatin, and etoposide for patients with previously untreated esophageal and gastroesophageal carcinomas. Cancer. 1999;85:2347–2351. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

