Niche metabolism in parasitic protozoa
- PMID: 16553311
- PMCID: PMC1626543
- DOI: 10.1098/rstb.2005.1756
Niche metabolism in parasitic protozoa
Abstract
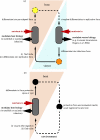
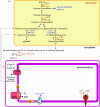
Complete or partial genome sequences have recently become available for several medically and evolutionarily important parasitic protozoa. Through the application of bioinformatics complete metabolic repertoires for these parasites can be predicted. For experimentally intractable parasites insight provided by metabolic maps generated in silico has been startling. At its more extreme end, such bioinformatics reckoning facilitated the discovery in some parasites of mitochondria remodelled beyond previous recognition, and the identification of a non-photosynthetic chloroplast relic in malarial parasites. However, for experimentally tractable parasites, mapping of the general metabolic terrain is only a first step in understanding how the parasite modulates its streamlined, yet still often puzzlingly complex, metabolism in order to complete life cycles within host, vector, or environment. This review provides a comparative overview and discussion of metabolic strategies used by several different parasitic protozoa in order to subvert and survive host defences, and illustrates how genomic data contribute to the elucidation of parasite metabolism.
Figures

References
-
- Abrahamsen M.S, et al. Complete genome sequence of the apicomplexan, Cryptosporidium parvum. Science. 2004;304:441–445. doi:10.1126/science.1094786 - DOI - PubMed
-
- Allen J.W, Ginger M.L, Ferguson S.J. Maturation of the unusual single-cysteine (XXXCH) mitochondrial c-type cytochromes found in trypanosomatids must occur through a novel biogenesis pathway. Biochem. J. 2004;383:537–542. doi:10.1042/BJ20040832 - DOI - PMC - PubMed
-
- Allocco J.J, Profous-Juchelka H, Myers R.W, Nare B, Schmatz D.M. Biosynthesis and catabolism of mannitol is developmentally regulated in the protozoan parasite Eimeria tenella. J. Parasitol. 1999;85:167–173. - PubMed
-
- Atwood J.A, III, Weatherly D.B, Minning T.A, Bundy B, Cavola C, Opperdoes F.R, Orlando R, Tarleton R.L. The Trypanosoma cruzi proteome. Science. 2005;309:473–476. doi:10.1126/science.1110289 - DOI - PubMed
-
- Bakker B.M, Mensonides F.I, Teusink B, van Hoek P, Michels P.A, Westerhoff H.V. Compartmentation protects trypanosomes from the dangerous design of glycolysis. Proc. Natl Acad. Sci. USA. 2000;97:2087–2092. doi:10.1073/pnas.030539197 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

