The Cinderella story of metabolic profiling: does metabolomics get to go to the functional genomics ball?
- PMID: 16553314
- PMCID: PMC1626538
- DOI: 10.1098/rstb.2005.1734
The Cinderella story of metabolic profiling: does metabolomics get to go to the functional genomics ball?
Abstract
To date most global approaches to functional genomics have centred on genomics, transcriptomics and proteomics. However, since a number of high-profile publications, interest in metabolomics, the global profiling of metabolites in a cell, tissue or organism, has been rapidly increasing. A range of analytical techniques, including 1H NMR spectroscopy, gas chromatography-mass spectrometry (GC-MS), liquid chromatography-mass spectrometry (LC-MS), Fourier Transform mass spectrometry (FT-MS), high performance liquid chromatography (HPLC) and electrochemical array (EC-array), are required in order to maximize the number of metabolites that can be identified in a matrix. Applications have included phenotyping of yeast, mice and plants, understanding drug toxicity in pharmaceutical drug safety assessment, monitoring tumour treatment regimes and disease diagnosis in human populations. These successes are likely to be built on as other analytical and bioinformatic approaches are developed to fully exploit the information obtained in metabolic profiles. To assist in this process, databases of metabolomic data will be necessary to allow the passage of information between laboratories. In this prospective review, the capabilities of metabolomics in the field of medicine will be assessed in an attempt to predict the impact this 'Cinderella approach' will have at the 'functional genomic ball'.
Figures
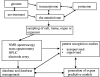
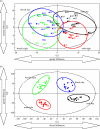
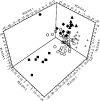
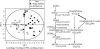


References
-
- Allen J, Davey H.M, Broadhurst D, Heald J.K, Rowland J.J, Oliver S.G, Kell D.B. High-throughput classification of yeast mutants for functional genomics using metabolic footprinting. Nat. Biotechnol. 2003;21:692–696. doi:10.1038/nbt823 - DOI - PubMed
-
- Bailey N.J, Sampson J, Hylands P.J, Nicholson J.K, Holmes E. Multi-component metabolic classification of commercial feverfew preparations via high-field 1H-NMR spectroscopy and chemometrics. Planta Med. 2002;68:734–738. doi:10.1055/s-2002-33793 - DOI - PubMed
-
- Bailey N.J, Oven M, Holmes E, Nicholson J.K, Zenk M.H. Metabolomic analysis of the consequences of cadmium exposure in Silene cucubalus cell cultures via 1H NMR spectroscopy and chemometrics. Phytochemistry. 2003;62:851–858. doi:10.1016/S0031-9422(02)00719-7 - DOI - PubMed
-
- Beckwith-Hall B.M, Nicholson J.K, Nicholls A.W, Foxall P.J, Lindon J.C, Connor S.C, Abdi M, Connelly J, Holmes E. Nuclear magnetic resonance spectroscopic and principal components anlysis investigations into biochemical effects of three model hepatotoxins. Chem. Res. Toxicol. 1998;11:260–272. doi:10.1021/tx9700679 - DOI - PubMed
-
- Bendall J.K, Heymes C, Wright T.J.F, Wheatcroft S, Grieve D.J, Shah A.M, Cave A.C. J. Mol. Cell. Cardiol. 2002;34:1325–1333. doi:10.1006/jmcc.2002.2083 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
