Nature's green revolution: the remarkable evolutionary rise of C4 plants
- PMID: 16553316
- PMCID: PMC1626541
- DOI: 10.1098/rstb.2005.1737
Nature's green revolution: the remarkable evolutionary rise of C4 plants
Abstract
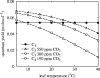
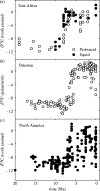
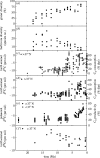
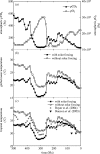
Plants with the C4 photosynthetic pathway dominate today's tropical savannahs and grasslands, and account for some 30% of global terrestrial carbon fixation. Their success stems from a physiological CO2-concentrating pump, which leads to high photosynthetic efficiency in warm climates and low atmospheric CO2 concentrations. Remarkably, their dominance of tropical environments was achieved in only the past 10 million years (Myr), less than 3% of the time that terrestrial plants have existed on Earth. We critically review the proposal that declining atmospheric CO2 triggered this tropical revolution via its effects on the photosynthetic efficiency of leaves. Our synthesis of the latest geological evidence from South Asia and North America suggests that this emphasis is misplaced. Instead, we find important roles for regional climate change and fire in South Asia, but no obvious environmental trigger for C4 success in North America. CO2-starvation is implicated in the origins of C4 plants 25-32 Myr ago, raising the possibility that the pathway evolved under more extreme atmospheric conditions experienced 10 times earlier. However, our geochemical analyses provide no evidence of the C4 mechanism at this time, although possible ancestral components of the C4 pathway are identified in ancient plant lineages. We suggest that future research must redress the substantial imbalance between experimental investigations and analyses of the geological record.
Figures





References
-
- Beerling D.J. Evolutionary responses of land plants to atmospheric CO2. In: Ehleringer J.R, Cerling T.E, Dearing D.M, editors. A history of atmospheric CO2 and its effects on plants, animals and ecosystems. Ecological studies. vol. 177. Springer; Berlin: 2005. pp. 114–132.
-
- Beerling D.J, Woodward F.I. Modelling the first 400 million years. Cambridge University Press; Cambridge, UK: 2001. Vegetation and the terrestrial carbon cycle.
-
- Bekker A, Holland H.D, Wang P.L, Rumble D, Stein H.J, Hannah J.L, Coetzee L.L, Beukes N.J. Dating the rise of atmospheric oxygen. Nature. 2004;427:117–120. doi:10.1038/nature02260 - DOI - PubMed
-
- Berner R.A. CO2 and O2. Oxford University Press; Oxford, UK: 2005. The Phanerozoic carbon cycle.
-
- Björkman O. Comparative photosynthetic CO2 exchange in higher plants. In: Hatch M.D, Osmond C.B, Slayter R.O, editors. Photosynthesis and photorespiration. Academic Press; San Diego, CA: 1971. pp. 18–32.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
