Chronic ethanol feeding increases activation of NADPH oxidase by lipopolysaccharide in rat Kupffer cells: role of increased reactive oxygen in LPS-stimulated ERK1/2 activation and TNF-alpha production
- PMID: 16554353
- PMCID: PMC1959405
- DOI: 10.1189/jlb.1005613
Chronic ethanol feeding increases activation of NADPH oxidase by lipopolysaccharide in rat Kupffer cells: role of increased reactive oxygen in LPS-stimulated ERK1/2 activation and TNF-alpha production
Abstract
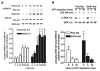
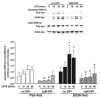
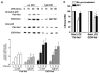
Reactive oxygen species (ROS) contribute to the development of chronic ethanol-induced liver injury. Although ROS modulate the activity of many signal transduction pathways, the molecular targets of ROS during ethanol exposure are not well understood. Here, we investigated whether specific ROS-sensitive signal transduction pathways contribute to increased tumor necrosis factor alpha (TNF-alpha) production by Kupffer cells after chronic ethanol feeding to rats. Lipopolysaccharide (LPS) rapidly increased ROS production, measured by dihydrorhodamine fluorescence, in Kupffer cells from ethanol- and pair-fed rats, and ROS production was 2.5-fold greater in ethanol-fed compared with pair-fed. Pretreatment with diphenyleneiodonium (DPI), which inhibits reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, normalized ROS production in Kupffer cells from ethanol-fed rats. LPS rapidly increased Rac1-guanosinetriphosphatase (GTPase) activity and p67(phox) translocation to the plasma membrane in Kupffer cells from pair-fed rats. After ethanol feeding, Rac1-GTPase activity was already increased over pair-fed at baseline and remained elevated over pair-fed after LPS stimulation. Further, LPS-stimulated p67(phox) translocation to the plasma membrane was enhanced after chronic ethanol feeding. LPS-stimulated extracellular signal-regulated kinase (ERK)1/2 and p38 phosphorylation, two signaling pathways regulated by ROS, were increased twofold in Kupffer cells from ethanol-fed rats compared with pair-fed controls. However, only LPS-stimulated ERK1/2 phosphorylation was inhibited by DPI, which also reduced LPS-stimulated TNF-alpha production in Kupffer cells from pair- and ethanol-fed rats. These results demonstrate that chronic ethanol feeding increases LPS-stimulated NADPH oxidase-dependent production of ROS in Kupffer cells. Further, ERK1/2 is an important target of NADPH oxidase-derived ROS in Kupffer cells, contributing to enhanced LPS-stimulated TNF-alpha production by Kupffer cells after chronic ethanol feeding.
Figures

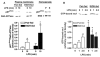

References
-
- Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med. 2000;343:1467–1476. - PubMed
-
- Reimund JM, Wittersheim C, Dumont S, Muller CD, Baumann R, Poindron P, Duclos B. Mucosal inflammatory cytokine production by intestinal biopsies in patients with ulcerative colitis and Crohn’s disease. J Clin Immunol. 1996;16:144–150. - PubMed
-
- Thurman RG. Mechanisms of hepatic toxicity II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol. 1998;275:G605–G611. - PubMed
-
- Honchel R, Ray M, Marsano L, Cohen D, Lee E, Shedlofsky S, McClain CJ. Tumor necrosis factor in alcohol enhanced endotoxin liver injury. Alcohol Clin Exp Res. 1992;16:665–669. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

