Neuregulin 1-erbB signaling is necessary for normal myelination and sensory function
- PMID: 16554459
- PMCID: PMC6674097
- DOI: 10.1523/JNEUROSCI.3785-05.2006
Neuregulin 1-erbB signaling is necessary for normal myelination and sensory function
Abstract
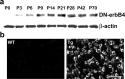
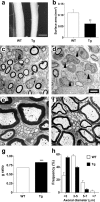
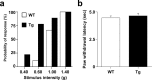
To investigate the role of erbB signaling in the interactions between peripheral axons and myelinating Schwann cells, we generated transgenic mice expressing a dominant-negative erbB receptor in these glial cells. Mutant mice have delayed onset of myelination, thinner myelin, shorter internodal length, and smaller axonal caliber in adulthood. Consistent with the morphological defects, transgenic mice also have slower nerve conduction velocity and defects in their responses to mechanical stimulation. Molecular analysis indicates that erbB signaling may contribute to myelin formation by regulating transcription of myelin genes. Analysis of sciatic nerves showed a reduction in the levels of expression of myelin genes in mutant mice. In vitro assays revealed that neuregulin-1 (NRG1) induces expression of myelin protein zero (P0). Furthermore, we found that the effects of NRG1 on P0 expression depend on the NRG1 isoform used. When NRG1 is presented to Schwann cells in the context of cell-cell contact, type III but not type I NRG1 regulates P0 gene expression. These results suggest that disruption of the NRG1-erbB signaling pathway could contribute to the pathogenesis of peripheral neuropathies with hypomyelination and neuropathic pain.
Figures



References
-
- Arroyo EJ, Scherer SS (2000). On the molecular architecture of myelinated fibers. Histochem Cell Biol 113:1–18. - PubMed
-
- Black JA, Liu S, Tanaka M, Cummins TR, Waxman SG (2004). Changes in the expression of tetrodotoxin-sensitive sodium channels within dorsal root ganglia neurons in inflammatory pain. Pain 108:237–247. - PubMed
-
- Buonanno A, Fischbach GD (2001). Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr Opin Neurobiol 11:287–296. - PubMed
-
- Carter GT, Jensen MP, Galer BS, Kraft GH, Crabtree LD, Beardsley RM, Abresch RT, Bird TD (1998). Neuropathic pain in Charcot-Marie-Tooth disease. Arch Phys Med Rehabil 79:1560–1564. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
