Human astrocytes are resistant to Fas ligand and tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis
- PMID: 16554480
- PMCID: PMC6674086
- DOI: 10.1523/JNEUROSCI.5572-05.2006
Human astrocytes are resistant to Fas ligand and tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis
Erratum in
- J Neurosci. 2006 May 3;26(18):4983
Abstract
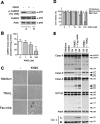
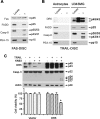
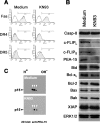
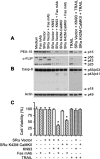
Human astrocytes express Fas yet are resistant to Fas-induced apoptosis. Here, we report that calcium/calmodulin-dependent protein kinase II (CaMKII) is constitutively activated in human astrocytes and protects the cells from apoptotic stimulation by Fas agonist. Once stimulated, Fas recruits Fas-associated death domain and caspase-8 for the assembly of the death-inducing signaling complex (DISC); however, caspase-8 cleavage is inhibited in the DISC. Inhibition of CaMKII kinase activity inhibits the expression of phosphoprotein enriched astrocytes-15 kDa/phosphoprotein enriched in diabetes (PEA-15/PED) and cellular Fas-associated death domain-like interleukin-1beta-converting enzyme-inhibitory protein (c-FLIP), thus releasing their inhibition of caspase-8 cleavage. Inhibition of PEA-15/PED or c-FLIP by small interfering RNA sensitizes human astrocytes to Fas-induced apoptosis. In contrast, inhibition of CaMKII, PEA-15, or c-FLIP does not affect the sensitivity of human astrocytes to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). TRAIL death receptors (DR4, DR5) are weakly expressed at mRNA, protein, and cell surface levels and thus fail to mediate the assembly of the DISC in human astrocytes. Overexpression of DR5 restores TRAIL signaling pathways and sensitizes the human astrocytes to TRAIL-induced apoptosis if CaMKII kinase activity or expression of PEA-15 and c-FLIP is inhibited; the results suggest that CaMKII-mediated pathways prevent TRAIL-induced apoptosis in human astrocytes under conditions in which TRAIL death receptors are upregulated. This study has therefore identified the molecular mechanisms that protect normal human astrocytes from apoptosis induced by Fas ligand and TRAIL.
Figures


References
-
- Araujo H, Danziger N, Cordier J, Glowinski J, Chneiweiss H (1993). Characterization of PEA-15, a major substrate for protein kinase C in astrocytes. J Biol Chem 268:5911–5920. - PubMed
-
- Ashkenazi A (2002). Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev Cancer 2:420–430. - PubMed
-
- Becher B, D’Souza SD, Troutt AB, Antel JP (1998). Fas expression on human fetal astrocytes without susceptibility to fas-mediated cytotoxicity. Neuroscience 84:627–634. - PubMed
-
- Cantarella G, Uberti D, Carsana T, Lombardo G, Bernardini R, Memo M (2003). Neutralization of TRAIL death pathway protects human neuronal cell line from beta-amyloid toxicity. Cell Death Differ 10:134–141. - PubMed
-
- Choi C, Benveniste EN (2004). Fas ligand/Fas system in the brain: regulator of immune and apoptotic responses. Brain Res Brain Res Rev 44:65–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
