Axonal ensheathment and septate junction formation in the peripheral nervous system of Drosophila
- PMID: 16554482
- PMCID: PMC6674093
- DOI: 10.1523/JNEUROSCI.5383-05.2006
Axonal ensheathment and septate junction formation in the peripheral nervous system of Drosophila
Abstract
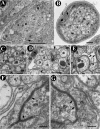
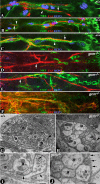
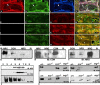
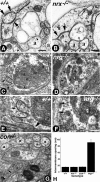
Axonal insulation is critical for efficient action potential propagation and normal functioning of the nervous system. In Drosophila, the underlying basis of nerve ensheathment is the axonal insulation by glial cells and the establishment of septate junctions (SJs) between glial cell membranes. However, the details of the cellular and molecular mechanisms underlying axonal insulation and SJ formation are still obscure. Here, we report the characterization of axonal insulation in the Drosophila peripheral nervous system (PNS). Targeted expression of tau-green fluorescent protein in the glial cells and ultrastructural analysis of the peripheral nerves allowed us to visualize the glial ensheathment of axons. We show that individual or a group of axons are ensheathed by inner glial processes, which in turn are ensheathed by the outer perineurial glial cells. SJs are formed between the inner and outer glial membranes. We also show that Neurexin IV, Contactin, and Neuroglian are coexpressed in the peripheral glial membranes and that these proteins exist as a complex in the Drosophila nervous system. Mutations in neurexin IV, contactin, and neuroglian result in the disruption of blood-nerve barrier function in the PNS, and ultrastructural analyses of the mutant embryonic peripheral nerves show loss of glial SJs. Interestingly, the murine homologs of Neurexin IV, Contactin, and Neuroglian are expressed at the paranodal SJs and play a key role in axon-glial interactions of myelinated axons. Together, our data suggest that the molecular machinery underlying axonal insulation and axon-glial interactions may be conserved across species.
Figures


References
-
- Anderson JM, Van Itallie CM, Fanning AS (2004). Setting up a selective barrier at the apical junction complex. Curr Opin Cell Biol 16:140–145. - PubMed
-
- Auld VJ, Fetter RD, Broadie K, Goodman CS (1995). Gliotactin, a novel transmembrane protein on peripheral glia, is required to form the blood-nerve barrier in Drosophila. Cell 81:757–767. - PubMed
-
- Bainton RJ, Tsai LT, Schwabe T, DeSalvo M, Gaul U, Heberlein U (2005). moody encodes two GPCRs that regulate cocaine behaviors and blood–brain barrier permeability in Drosophila. Cell 123:145–156. - PubMed
-
- Banerjee S, Sousa AD, Bhat MA (2006). Organization and function of septate junctions: an evolutionary perspective. Cell Biochem Biophys in press. - PubMed
-
- Baumgartner S, Littleton JT, Broadie K, Bhat MA, Harbecke R, Lengyel JA, Chiquet-Ehrismann R, Prokop A, Bellen HJ (1996). A Drosophila neurexin is required for septate junction and blood-nerve barrier formation and function. Cell 87:1059–1068. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
