An analog of a dipeptide-like structure of FK506 increases glial cell line-derived neurotrophic factor expression through cAMP response element-binding protein activated by heat shock protein 90/Akt signaling pathway
- PMID: 16554484
- PMCID: PMC6674092
- DOI: 10.1523/JNEUROSCI.5010-05.2006
An analog of a dipeptide-like structure of FK506 increases glial cell line-derived neurotrophic factor expression through cAMP response element-binding protein activated by heat shock protein 90/Akt signaling pathway
Abstract
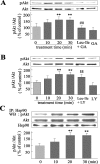
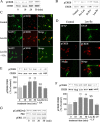
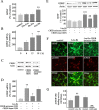
Glial cell line-derived neurotrophic factor (GDNF) is an important neurotrophic factor that has therapeutic implications for neurodegenerative disorders. We previously showed that leucine-isoleucine (Leu-Ile), an analog of a dipeptide-like structure of FK506 (tacrolimus), induces GDNF expression both in vivo and in vitro. In this investigation, we sought to clarify the cellular mechanisms underlying the GDNF-inducing effect of this dipeptide. Leu-Ile transport was investigated using fluorescein isothiocyanate-Leu-Ile in cultured neurons, and the results showed the transmembrane mobility of this dipeptide. By liquid chromatography-mass spectrometry and quartz crystal microbalance assay, we identified heat shock cognate protein 70 as a protein binding specifically to Leu-Ile, and molecular modeling showed that the ATPase domain is the predicted binding site. Leu-Ile stimulated Akt phosphorylation, which was attenuated significantly by heat shock protein 90 (Hsp90) inhibitor geldanamycin (GA). Moreover, enhanced interaction between phosphorylated Akt and Hsp90 was detected by immunoprecipitation. Leu-Ile elicited an increase in cAMP response element binding protein (CREB) phosphorylation, which was inhibited by GA, indicating that CREB is a downstream target of Hsp90/Akt signaling. Leu-Ile elevated the levels of GDNF mRNA and protein expression, whereas inhibition of CREB blocked such effects. Leu-Ile promoted the binding activity of phosphorylated CREB with cAMP response element. These findings show that CREB plays a key role in transcriptional regulation of GDNF expression induced by Leu-Ile. In conclusion, Leu-Ile activates Hsp90/Akt/CREB signaling, which contributes to the upregulation of GDNF expression. It may represent a novel lead compound for the treatment of dopaminergic neurons or motoneuron diseases.
Figures




References
-
- Airaksinen MS, Saarma M (2002). The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci 3:383–394. - PubMed
-
- Baecker PA, Lee WH, Verity AN, Eglen RM, Johnson RM (1999). Characterization of a promoter for the human glial cell line-derived neurotrophic factor gene. Mol Brain Res 69:209–222. - PubMed
-
- Basso AD, Solit DB, Chiosis G, Giri B, Tsichlis P, Rosen N (2002). Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J Biol Chem 277:39858–39866. - PubMed
-
- Brunet A, Datta SR, Greenberg ME (2001). Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol 11:297–305. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
