Improvements in siRNA properties mediated by 2'-deoxy-2'-fluoro-beta-D-arabinonucleic acid (FANA)
- PMID: 16554553
- PMCID: PMC1409815
- DOI: 10.1093/nar/gkl033
Improvements in siRNA properties mediated by 2'-deoxy-2'-fluoro-beta-D-arabinonucleic acid (FANA)
Abstract
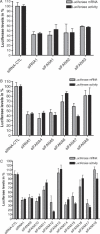
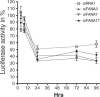
RNA interference (RNAi) has emerged recently as an efficient mechanism for specific gene silencing. Short double-stranded small interfering RNAs (siRNAs) are now widely used for cellular or drug target validation; however, their use for silencing clinically relevant genes in a therapeutic setting remains problematic because of their unfavourable metabolic stability and pharmacokinetic properties. To address some of these concerns, we have investigated the properties of siRNA modified with 2'-deoxy-2'-fluoro-beta-d-arabinonucleotide units (araF-N or FANA units). Here we provide evidence that these modified siRNAs are compatible with the intracellular RNAi machinery and can mediate specific degradation of target mRNA. We also show that the incorporation of FANA units into siRNA duplexes increases activity and substantially enhances serum stability of the siRNA. A fully modified sense 2'-deoxy-2'-fluoro-beta-D-arabinonucleic acid (FANA) strand when hybridized to an antisense RNA (i.e. FANA/RNA hybrid) was shown to be 4-fold more potent and had longer half-life in serum (approximately 6 h) compared with an unmodified siRNA (<15 min). While incorporation of FANA units is well tolerated throughout the sense strand of the duplex, modifications can also be included at the 5' or 3' ends of the antisense strand, in striking contrast to other commonly used chemical modifications. Taken together, these results offer preliminary evidence of the therapeutic potential of FANA modified siRNAs.
Figures



References
-
- Opalinska J.B., Gewirtz A.M. Nucleic-acid therapeutics: basic principles and recent applications. Nature Rev. 2002;1:1–10. - PubMed
-
- Scherer L.J., Rossi J.J. Approaches for sequence-specific knockdown of mRNA. Nat. Biotechnol. 2003;21:1457–1465. - PubMed
-
- Fire A., Xu S., Montgomery M.K., Kostas S.A., Driver S.E., Mello C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

