A voltage-gated proton-selective channel lacking the pore domain
- PMID: 16554753
- PMCID: PMC4084761
- DOI: 10.1038/nature04700
A voltage-gated proton-selective channel lacking the pore domain
Abstract
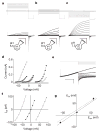
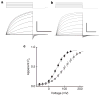
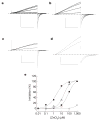
Voltage changes across the cell membrane control the gating of many cation-selective ion channels. Conserved from bacteria to humans, the voltage-gated-ligand superfamily of ion channels are encoded as polypeptide chains of six transmembrane-spanning segments (S1-S6). S1-S4 functions as a self-contained voltage-sensing domain (VSD), in essence a positively charged lever that moves in response to voltage changes. The VSD 'ligand' transmits force via a linker to the S5-S6 pore domain 'receptor', thereby opening or closing the channel. The ascidian VSD protein Ci-VSP gates a phosphatase activity rather than a channel pore, indicating that VSDs function independently of ion channels. Here we describe a mammalian VSD protein (H(V)1) that lacks a discernible pore domain but is sufficient for expression of a voltage-sensitive proton-selective ion channel activity. H(v)1 currents are activated at depolarizing voltages, sensitive to the transmembrane pH gradient, H+-selective, and Zn2+-sensitive. Mutagenesis of H(v)1 identified three arginine residues in S4 that regulate channel gating and two histidine residues that are required for extracellular inhibition of H(v)1 by Zn2+. H(v)1 is expressed in immune tissues and manifests the characteristic properties of native proton conductances (G(vH+)). In phagocytic leukocytes, G(vH+) are required to support the oxidative burst that underlies microbial killing by the innate immune system. The data presented here identify H(v)1 as a long-sought voltage-gated H+ channel and establish H(v)1 as the founding member of a family of mammalian VSD proteins.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
-
- Yu FH, Catterall WA. The VGL-chanome: a protein superfamily specialized for electrical signaling and ionic homeostasis. Sci STKE. 2004;2004:re15. - PubMed
-
- Long SB, Campbell EB, Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. - PubMed
-
- Murata Y, Iwasaki H, Sasaki M, Inaba K, Okamura Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 2005;435:1239–1243. - PubMed
-
- DeCoursey TE. Voltage-gated proton channels and other proton transfer pathways. Physiol Rev. 2003;83:475–579. - PubMed
-
- DeCoursey TE, Morgan D, Cherny VV. The voltage dependence of NADPH oxidase reveals why phagocytes need proton channels. Nature. 2003;422:531–534. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

