The ABC protein turned chloride channel whose failure causes cystic fibrosis
- PMID: 16554808
- PMCID: PMC2720541
- DOI: 10.1038/nature04712
The ABC protein turned chloride channel whose failure causes cystic fibrosis
Abstract
CFTR chloride channels are encoded by the gene mutated in patients with cystic fibrosis. These channels belong to the superfamily of ABC transporter ATPases. ATP-driven conformational changes, which in other ABC proteins fuel uphill substrate transport across cellular membranes, in CFTR open and close a gate to allow transmembrane flow of anions down their electrochemical gradient. New structural and biochemical information from prokaryotic ABC proteins and functional information from CFTR channels has led to a unifying mechanism explaining those ATP-driven conformational changes.
Figures
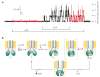

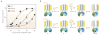

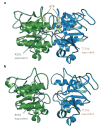
References
-
- Rommens JM, et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245:1059–1065. - PubMed
-
- Riordan JR, et al. Identification of the cystic fibrosis gene: Cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. - PubMed
-
- Quinton PM. Chloride impermeability in cystic fibrosis. Nature. 1983;301:421–422. - PubMed
-
- Schoumacher RA, et al. Phosphorylation fails to activate chloride channels from cystic fibrosis airway cells. Nature. 1987;330:752–754. - PubMed
-
- Li M, et al. Cyclic AMP-dependent protein kinase opens chloride channels in normal but not cystic fibrosis airway epithelium. Nature. 1988;331:358–360. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

