Age-dependent genetic variance in a life-history trait in the mute swan
- PMID: 16555791
- PMCID: PMC1560027
- DOI: 10.1098/rspb.2005.3294
Age-dependent genetic variance in a life-history trait in the mute swan
Abstract
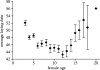
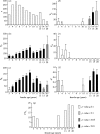
Genetic variance in characters under natural selection in natural populations determines the way those populations respond to that selection. Whether populations show temporal and/or spatial constancy in patterns of genetic variance and covariance is regularly considered, as this will determine whether selection responses are constant over space and time. Much less often considered is whether characters show differing amounts of genetic variance over the life-history of individuals. Such age-specific variation, if present, has important potential consequences for the force of natural selection and for understanding the causes of variation in quantitative characters. Using data from a long-term study of the mute swan Cygnus olor, we report the partitioning of phenotypic variance in timing of breeding (subject to strong natural selection) into component parts over 12 different age classes. We show that the additive genetic variance and heritability of this trait are strongly age-dependent, with higher additive genetic variance present in young and, particularly, old birds, but little evidence of any genetic variance for birds of intermediate ages. These results demonstrate that age can have a very important influence on the components of variation of characters in natural populations, and consequently that separate age classes cannot be assumed to be equivalent, either with respect to their evolutionary potential or response.
Figures
References
-
- Albuquerque L.G, Meyer K. Estimates of covariance functions for growth from birth to 630 days of age in Nelore cattle. J. Anim. Sci. 2001a;79:2776–2789. - PubMed
-
- Albuquerque L.G, Meyer K. Estimates of direct and maternal genetic effects for weights from birth to 600 days of age in Nelore cattle. J. Anim. Breed. Genet. 2001b;118:83–92. 10.1046/j.1439-0388.2001.00279.x - DOI
-
- Arnold S.J, Wade M.J. On the measurement of natural and sexual selection: theory. Evolution. 1984;38:709–719. - PubMed
-
- Atchley W.R. Ontogeny, timing of development, and genetic variance–covariance structure. Am. Nat. 1984;123:519–540. 10.1086/284220 - DOI
-
- Badyaev A.V, Martin T.E. Individual variation in growth trajectories: phenotypic and genetic correlations in ontogeny of the house finch (Carpodacus mexicanus) J. Evol. Biol. 2000;13:290–301. 10.1046/j.1420-9101.2000.00172.x - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

