Adrenal responses to low dose synthetic ACTH (Synacthen) in children receiving high dose inhaled fluticasone
- PMID: 16556614
- PMCID: PMC2066000
- DOI: 10.1136/adc.2005.087247
Adrenal responses to low dose synthetic ACTH (Synacthen) in children receiving high dose inhaled fluticasone
Abstract
Background and aims: Clinical adrenal insufficiency has been reported with doses of inhaled fluticasone proprionate (FP) > 400 microg/day, the maximum dose licensed for use in children with asthma. Following two cases of serious adrenal insufficiency (one fatal) attributed to FP, adrenal function was evaluated in children receiving FP outwith the licensed dose.
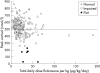
Methods: Children recorded as prescribed FP > or = 500 microg/day were invited to attend for assessment. Adrenal function was measured using the low dose Synacthen test (500 ng/1.73 m2 intravenously) and was categorised as: biochemically normal (peak cortisol response > 500 nmol/l); impaired (peak cortisol < or = 500 nmol/l); or flat (peak cortisol < or = 500 nmol/l with increment of < 200 nmol/l and basal morning cortisol < 200 nmol/l).
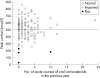
Results: A total of 422 children had been receiving FP alone or in combination with salmeterol; 202 were not investigated (137 FP within license; 24 FP discontinued); 220 attended and 217 (age 2.6-19.3 years) were successfully tested. Of 194 receiving FP > or = 500 microg/day, six had flat responses, 82 impaired responses, 104 were normal, and in 2 the LDST was unsuccessful. Apart from the index child, the other five with flat responses were asymptomatic; a further child with impairment (peak cortisol 296 nmol/l) had encephalopathic symptoms with borderline hypoglycaemia during an intercurrent illness. The six with flat responses and the symptomatic child were all receiving FP doses of > or = 1000 microg/day.
Conclusion: Overall, flat adrenal responses in association with FP occurred in 2.8% of children tested, all receiving > or = 1000 microg/day, while impaired responses were seen in 39.6%. Children on above licence FP doses should have adrenal function monitoring as well as a written plan for emergency steroid replacement.
Conflict of interest statement
Competing interests: JYP has received financial support for clinical trials, attending conferences and postgraduate meetings, and from companies who make inhaled steroids, including GlaxoSmithKline, AstraZeneca, 3M Healthcare, and Novartis. His spouse has shares in GlaxoSmithKline, which makes fluticasone.
Comment in
-
Very high dose inhaled corticosteroids: panacea or poison?Arch Dis Child. 2006 Oct;91(10):802-4. doi: 10.1136/adc.2006.098616. Arch Dis Child. 2006. PMID: 16990353 Free PMC article. Review.
-
Compliance with inhaled corticosteroids is important when considering adrenal suppression.Arch Dis Child. 2007 Apr;92(4):372. doi: 10.1136/adc.2006.111732. Arch Dis Child. 2007. PMID: 17376955 Free PMC article. No abstract available.
References
-
- Lipworth B J. Systemic adverse effects of inhaled corticosteroid therapy: a systematic review and meta‐analysis. Arch Intern Med 1999159941–955. - PubMed