A specific pattern of phosphodiesterases controls the cAMP signals generated by different Gs-coupled receptors in adult rat ventricular myocytes
- PMID: 16556871
- PMCID: PMC2099453
- DOI: 10.1161/01.RES.0000218493.09370.8e
A specific pattern of phosphodiesterases controls the cAMP signals generated by different Gs-coupled receptors in adult rat ventricular myocytes
Abstract
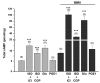
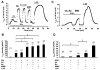
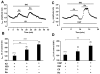
Compartmentation of cAMP is thought to generate the specificity of Gs-coupled receptor action in cardiac myocytes, with phosphodiesterases (PDEs) playing a major role in this process by preventing cAMP diffusion. We tested this hypothesis in adult rat ventricular myocytes by characterizing PDEs involved in the regulation of cAMP signals and L-type Ca2+ current (I(Ca,L)) on stimulation with beta1-adrenergic receptors (beta1-ARs), beta2-ARs, glucagon receptors (Glu-Rs) and prostaglandin E1 receptors (PGE1-Rs). All receptors but PGE1-R increased total cAMP, and inhibition of PDEs with 3-isobutyl-1-methylxanthine strongly potentiated these responses. When monitored in single cells by high-affinity cyclic nucleotide-gated (CNG) channels, stimulation of beta1-AR and Glu-R increased cAMP, whereas beta2-AR and PGE1-R had no detectable effect. Selective inhibition of PDE3 by cilostamide and PDE4 by Ro 20-1724 potentiated beta1-AR cAMP signals, whereas Glu-R cAMP was augmented only by PD4 inhibition. PGE1-R and beta2-AR generated substantial cAMP increases only when PDE3 and PDE4 were blocked. For all receptors except PGE1-R, the measurements of I(Ca,L) closely matched the ones obtained with CNG channels. Indeed, PDE3 and PDE4 controlled beta1-AR and beta2-AR regulation of I(Ca,L), whereas only PDE4 controlled Glu-R regulation of I(Ca,L) thus demonstrating that receptor-PDE coupling has functional implications downstream of cAMP. PGE1 had no effect on I(Ca,L) even after blockade of PDE3 or PDE4, suggesting that other mechanisms prevent cAMP produced by PGE1 to diffuse to L-type Ca2+ channels. These results identify specific functional coupling of individual PDE families to Gs-coupled receptors as a major mechanism enabling cardiac cells to generate heterogeneous cAMP signals in response to different hormones.
Figures




References
-
- Xiao RP, Lakatta EG. Beta 1-adrenoceptor stimulation and beta 2-adrenoceptor stimulation differ in their effects on contraction, cytosolic Ca2+, and Ca2+ current in single rat ventricular cells. Circ Res. 1993;73:286–300. - PubMed
-
- Bartel S, Krause EG, Wallukat G, Karczewski P. New insights into beta2-adrenoceptor signaling in the adult rat heart. Cardiovasc Res. 2003;57:694–703. - PubMed
-
- Kuznetsov V, Pak E, Robinson RB, Steinberg SF. Beta 2-adrenergic receptor actions in neonatal and adult rat ventricular myocytes. Circ Res. 1995;76:40–52. - PubMed
-
- Farah AE. Glucagon and the circulation. Pharmacol Rev. 1983;35:181–217. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

