Postinfarct cytokine therapy regenerates cardiac tissue and improves left ventricular function
- PMID: 16556872
- PMCID: PMC3652380
- DOI: 10.1161/01.RES.0000218454.76784.66
Postinfarct cytokine therapy regenerates cardiac tissue and improves left ventricular function
Abstract
We systematically investigated the comparative efficacy of three different cytokine regimens, administered after a reperfused myocardial infarction, in regenerating cardiac tissue and improving left ventricular (LV) function. Wild-type (WT) mice underwent a 30-minute coronary occlusion followed by reperfusion and received vehicle, granulocyte colony-stimulating factor (G-CSF)+Flt-3 ligand (FL), G-CSF+stem cell factor (SCF), or G-CSF alone starting 4 hours after reperfusion. In separate experiments, chimeric mice generated by reconstitution of radioablated WT mice with bone marrow from enhanced green fluorescent protein (EGFP) transgenic mice underwent identical protocols. Mice were euthanized 5 weeks later. Echocardiographically, LV function was improved in G-CSF+FL- and G-CSF+SCF-treated but not in G-CSF-treated mice, whereas LV end-diastolic dimensions were smaller in all three groups. Morphometrically, cytokine-treated hearts had smaller LV diameter and volume. Numerous EGFP-positive cardiomyocytes, capillaries, and arterioles were noted in the infarcted region in cytokine-treated chimeric mice treated with G-CSF+FL or G-CSF+SCF, but the numbers were much smaller in G-CSF-treated mice. G-CSF+FL therapy mobilized bone marrow-derived cells exhibiting increased expression of surface antigens (CD62L and CD11a) that facilitate homing. We conclude that postinfarct cytokine therapy with G-CSF+FL or G-CSF+SCF limits adverse LV remodeling and improves LV performance by promoting cardiac regeneration and probably also by exerting other beneficial actions unrelated to regeneration, and that G-CSF alone is less effective.
Figures

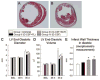


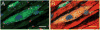
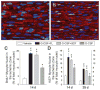


Comment in
-
Synergism of hematopoietic cytokines for infarct repair.Circ Res. 2006 Apr 28;98(8):990-2. doi: 10.1161/01.RES.0000222024.14452.7b. Circ Res. 2006. PMID: 16645148 No abstract available.
References
-
- Fukuhara S, Tomita S, Nakatani T, Ohtsu Y, Ishida M, Yutani C, Kitamura S. G-CSF promotes bone marrow cells to migrate into infarcted mice heart, and differentiate into cardiomyocytes. Cell Transplant. 2004;13:741–748. - PubMed
-
- Kawada H, Fujita J, Kinjo K, Matsuzaki Y, Tsuma M, Miyatake H, Muguruma Y, Tsuboi K, Itabashi Y, Ikeda Y, Ogawa S, Okano H, Hotta T, Ando K, Fukuda K. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood. 2004;104:3581–3587. - PubMed
-
- Wollert KC, Drexler H. Clinical applications of stem cells for the heart. Circ Res. 2005;96:151–163. - PubMed
-
- Deten A, Volz HC, Clamors S, Leiblein S, Briest W, Marx G, Zimmer HG. Hematopoietic stem cells do not repair the infarcted mouse heart. Cardiovasc Res. 2005;65:52–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 HL055757/HL/NHLBI NIH HHS/United States
- R01 HL076794/HL/NHLBI NIH HHS/United States
- HL-68088/HL/NHLBI NIH HHS/United States
- HL-70897/HL/NHLBI NIH HHS/United States
- R01 HL072410/HL/NHLBI NIH HHS/United States
- HL-55757/HL/NHLBI NIH HHS/United States
- HL-63442/HL/NHLBI NIH HHS/United States
- HL-72410/HL/NHLBI NIH HHS/United States
- R01 HL068088/HL/NHLBI NIH HHS/United States
- HL-78825/HL/NHLBI NIH HHS/United States
- R01 HL055757/HL/NHLBI NIH HHS/United States
- R01 HL070897/HL/NHLBI NIH HHS/United States
- DK-069766/DK/NIDDK NIH HHS/United States
- P01 HL078825/HL/NHLBI NIH HHS/United States
- HL-76794/HL/NHLBI NIH HHS/United States
- R01 DK069766/DK/NIDDK NIH HHS/United States
- R01 HL063442/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

