Discriminatory RNP remodeling by the DEAD-box protein DED1
- PMID: 16556937
- PMCID: PMC1440896
- DOI: 10.1261/rna.2323406
Discriminatory RNP remodeling by the DEAD-box protein DED1
Abstract
DExH/D proteins catalyze NTP-driven rearrangements of RNA and RNA-protein complexes during most aspects of RNA metabolism. Although the vast majority of DExH/D proteins displays virtually no sequence-specificity when remodeling RNA complexes in vitro, the enzymes clearly distinguish between a large number of RNA and RNP complexes in a physiological context. It is unknown how this discrimination between potential substrates is achieved. Here we show one possible way by which a non-sequence specific DExH/D protein can discriminately remodel similar RNA complexes. We have measured in vitro the disassembly of model RNPs by two distinct DExH/D proteins, DED1 and NPH-II. Both enzymes displace the U1 snRNP from a tightly bound RNA in an active, ATP-dependent fashion. However, DED1 cannot actively displace the protein U1A from its binding site, whereas NPH-II can. The dissociation rate of U1A dictates the rate by which DED1 remodels RNA complexes with U1A bound. We further show that DED1 disassembles RNA complexes with slightly altered U1A binding sites at different rates, but only when U1A is bound to the RNA. These findings suggest that the "inability" to actively displace other proteins from RNA can provide non-sequence specific DExH/D proteins with the capacity to disassemble similar RNA complexes in a discriminatory fashion. In addition, our study illuminates possible mechanisms for protein displacement by DExH/D proteins.
Figures

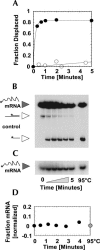
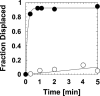
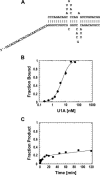


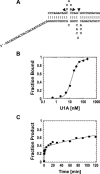

References
-
- Burgess S., Guthrie C. Beat the clock: Paradigms for NTPases in the maintenance of biological fidelity. Trends Biochem. Sci. 1993;18:381–384. - PubMed
-
- Fairman M., Maroney P.A., Wang W., Bowers H., Gollnick P., Nilsen T.W., Jankowsky E. Protein displacement by DExH/D RNA helicases without duplex unwinding. Science. 2004;304:730–734. - PubMed
-
- Iost I., Dreyfus M., Linder P. Ded1p, a DEAD-box protein required for translation initiation in Saccharomyces cerevisiae, is an RNA helicase. J. Biol. Chem. 1999;274:17677–17683. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
