Induction and blockage of oligodendrogenesis by differently activated microglia in an animal model of multiple sclerosis
- PMID: 16557302
- PMCID: PMC1409740
- DOI: 10.1172/JCI26836
Induction and blockage of oligodendrogenesis by differently activated microglia in an animal model of multiple sclerosis
Abstract
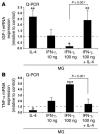
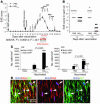
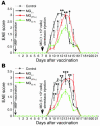
The role of activated microglia (MG) in demyelinating neurodegenerative diseases such as multiple sclerosis is controversial. Here we show that high, but not low, levels of IFN-gamma (a cytokine associated with inflammatory autoimmune diseases) conferred on rodent MG a phenotype that impeded oligodendrogenesis from adult neural stem/progenitor cells. IL-4 reversed the impediment, attenuated TNF-alpha production, and overcame blockage of IGF-I production caused by IFN-gamma. In rodents with acute or chronic EAE, injection of IL-4-activated MG into the cerebrospinal fluid resulted in increased oligodendrogenesis in the spinal cord and improved clinical symptoms. The newly formed oligodendrocytes were spatially associated with MG expressing MHC class II proteins and IGF-I. These results point to what we believe to be a novel role for MG in oligodendrogenesis from the endogenous stem cell pool.
Figures

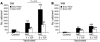



References
-
- Eriksson P.S., et al. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4:1313–1317. - PubMed
-
- Monje M.L., Toda H., Palmer T.D. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. - PubMed
-
- Hartung H.P., et al. Inflammatory mediators in demyelinating disorders of the CNS and PNS. . J. Neuroimmunol. 1992;40:197–210. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

