Synthetic multivalent ligands as probes of signal transduction
- PMID: 16557636
- PMCID: PMC2842921
- DOI: 10.1002/anie.200502794
Synthetic multivalent ligands as probes of signal transduction
Abstract
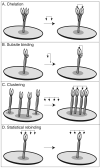
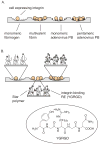
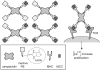
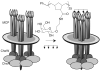
Cell-surface receptors acquire information from the extracellular environment and coordinate intracellular responses. Many receptors do not operate as individual entities, but rather as part of dimeric or oligomeric complexes. Coupling the functions of multiple receptors may endow signaling pathways with the sensitivity and malleability required to govern cellular responses. Moreover, multireceptor signaling complexes may provide a means of spatially segregating otherwise degenerate signaling cascades. Understanding the mechanisms, extent, and consequences of receptor co-localization and interreceptor communication is critical; chemical synthesis can provide compounds to address the role of receptor assembly in signal transduction. Multivalent ligands can be generated that possess a variety of sizes, shapes, valencies, orientations, and densities of binding elements. This Review focuses on the use of synthetic multivalent ligands to characterize receptor function.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

