Prognostic value of monitoring tumour markers CA 15-3 and CEA during fulvestrant treatment
- PMID: 16563172
- PMCID: PMC1435763
- DOI: 10.1186/1471-2407-6-81
Prognostic value of monitoring tumour markers CA 15-3 and CEA during fulvestrant treatment
Abstract
Background: At many centres tumour markers are used to detect disease recurrence and to monitor response to therapy in patients with advanced disease, although the real value of serial observation of marker levels remains disputed. In this study, we evaluated the prognostic value of tumour markers for predicting response (partial response [PR], stable disease [SD] > or = 6 months), de novo disease progression (PD) and secondary PD in patients receiving fulvestrant ('Faslodex') 250 mg/month for the treatment of metastatic breast cancer (MBC).
Methods: Changes in cancer antigen 15-3 (CA 15-3) and carcinoembryonic antigen (CEA) were prospectively monitored (monthly) and were also evaluated for the 3 months preceding secondary PD. Data from 67 patients with previously treated MBC participating in a Compassionate Use Programme were analysed.
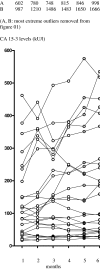
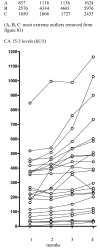
Results: In patients with a PR (n = 7 [10.4%]), a non-significant increase in CA 15-3 occurred during the first 6 months of treatment; CEA was significantly reduced (P = 0.0165). In patients with SD >/= 6 months (n = 28 [41.8%]), both CA 15-3 (P < 0.0001) and CEA (P = 0.0399) levels increased significantly after 6 months treatment. In those experiencing de novo PD (n = 32 [47.8%]), CA 15-3 increased significantly (P < 0.0001) after 4 months; CEA also increased significantly (P = 0.0002) during the same time period. Both CA 15-3 (P < 0.0001) and CEA (P < 0.0001) increased significantly in the 3 months preceding secondary PD.
Conclusion: CA 15-3 increases in patients progressing on fulvestrant but may also increase in those experiencing clinical benefit; this should not be taken as a sign of PD without verification. Overall, both CA 15-3 and CEA appear to be poor prognostic markers for determining progression in patients receiving fulvestrant.
Figures

References
-
- Massacesi C, Rocchi MB, Marcucci F, Pilone A, Galeazzi M, Bonsignori M. Serum tumor markers may precede instrumental response to chemotherapy in patients with metastatic cancer. Int J Biol Markers. 2003;18:295–300. - PubMed
-
- Pompecki R, Schroder G, Garbrecht M, Frahm H. [Carcinoembryonic antigens (CEA) in patients with metastatic breast cancer under endocrine and therapeutic treatment (author's transl)] Dtsch Med Wochenschr. 1978;103:620–622. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

