An anion antiporter model of prestin, the outer hair cell motor protein
- PMID: 16565043
- PMCID: PMC1459494
- DOI: 10.1529/biophysj.105.073254
An anion antiporter model of prestin, the outer hair cell motor protein
Abstract
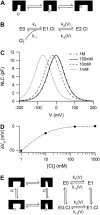
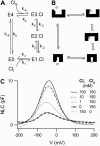
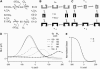
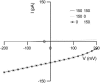
Cochlear amplification in mammalian hearing relies on an active mechanical feedback process generated by outer hair cells, driven by a protein, prestin (SLC26A5), in the lateral membrane. We have used kinetic models to understand the mechanism by which prestin might function. We show that the two previous hypotheses of prestin, which assume prestin cannot operate as a transporter, are insufficient to explain previously published data. We propose an alternative model of prestin as an electrogenic anion exchanger, exchanging one Cl(-) ion for one divalent or two monovalent anions. This model can reproduce the key aspects of previous experimental observations. The experimentally observed charge movements are produced by the translocation of one Cl(-) ion combined with intrinsic positively charged residues, while the transport of the counteranion is electroneutral. We tested the model with measurements of the Cl(-) dependence of charge movement, using SO(4)(2-) to replace Cl(-). The data was compatible with the predictions of the model, suggesting that prestin does indeed function as a transporter.
Figures


References
-
- Dallos, P., and D. Harris. 1978. Properties of auditory nerve responses in absence of outer hair cells. J. Neurophysiol. 41:365–383. - PubMed
-
- Liberman, M. C., J. Gao, D. Z. He, X. Wu, S. Jia, and J. Zuo. 2002. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature. 419:300–304. - PubMed
-
- Ashmore, J. F. 1990. Forward and reverse transduction in the mammalian cochlea. Neurosci. Res. Suppl. 12:S39–S50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

