Uncoupling store-operated Ca2+ entry and altered Ca2+ release from sarcoplasmic reticulum through silencing of junctophilin genes
- PMID: 16565048
- PMCID: PMC1471867
- DOI: 10.1529/biophysj.105.076570
Uncoupling store-operated Ca2+ entry and altered Ca2+ release from sarcoplasmic reticulum through silencing of junctophilin genes
Abstract
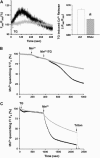
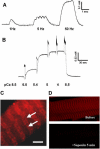
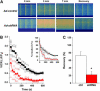
Junctophilin (JP) mediates the close contact between cell surface and intracellular membranes in muscle cells ensuring efficient excitation-contraction coupling. Here we demonstrate that disruption of triad junction structure formed by the transverse tubular (TT) invagination of plasma membrane and terminal cisternae of sarcoplasmic reticulum (SR) by reduction of JP expression leads to defective Ca2+ homeostasis in muscle cells. Using adenovirus with small hairpin interference RNA (shRNA) against both JP1 and JP2 genes, we could achieve acute suppression of JPs in skeletal muscle fibers. The shRNA-treated muscles exhibit deformed triad junctions and reduced store-operated Ca2+ entry (SOCE), which is likely due to uncoupled retrograde signaling from SR to TT. Knockdown of JP also causes a reduction in SR Ca2+ storage and altered caffeine-induced Ca2+ release, suggesting an orthograde regulation of the TT membrane on the SR Ca2+ release machinery. Our data demonstrate that JPs play an important role in controlling overall intracellular Ca2+ homeostasis in muscle cells. We speculate that altered expression of JPs may underlie some of the phenotypic changes associated with certain muscle diseases and aging.
Figures





References
-
- Dirksen, R. T. 2002. Bi-directional coupling between dihydropyridine receptors and ryanodine receptors. Front. Biosci. 7:d659–d670. - PubMed
-
- Ma, J., and Z. Pan. 2003. Junctional membrane structure and store operated calcium entry in muscle cells. Front. Biosci. 8:d242–d255. - PubMed
-
- Franzini-Armstrong, C., and A. O. Jorgensen. 1994. Structure and development of E-C coupling units in skeletal muscle. Annu. Rev. Physiol. 56:509–534. - PubMed
-
- Takeshima, H., S. Komazaki, M. Nishi, M. Iino, and K. Kangawa. 2000. Junctophilins: a novel family of junctional membrane complex proteins. Mol. Cell. 6:11–22. - PubMed
-
- Nishi, M., H. Sakagami, S. Komazaki, H. Kondo, and H. Takeshima. 2003. Coexpression of junctophilin type 3 and type 4 in brain. Brain Res. Mol. Brain Res. 118:102–110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

