Channel density regulation of firing patterns in a cortical neuron model
- PMID: 16565052
- PMCID: PMC1471851
- DOI: 10.1529/biophysj.105.077032
Channel density regulation of firing patterns in a cortical neuron model
Abstract
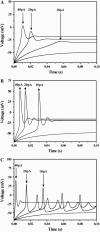
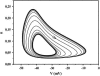
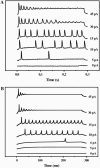
Modifying the density and distribution of ion channels in a neuron (by natural up- and downregulation or by pharmacological intervention or by spontaneous mutations) changes its activity pattern. In this investigation we analyzed how the impulse patterns are regulated by the density of voltage-gated channels in a neuron model based on voltage-clamp measurements of hippocampal interneurons. At least three distinct oscillatory patterns, associated with three distinct regions in the Na-K channel density plane, were found. A stability analysis showed that the different regions are characterized by saddle-node, double-orbit, and Hopf-bifurcation threshold dynamics, respectively. Single, strongly graded action potentials occur in an area outside the oscillatory regions, but less graded action potentials occur together with repetitive firing over a considerable range of channel densities. The relationship found here between channel densities and oscillatory behavior may partly explain the difference between the principal spiking patterns previously described for crab axons (class 1 and 2) and cortical neurons (regular firing and fast spiking).
Figures






References
-
- Hutcheon, B., and Y. Yarom. 2000. Resonance, oscillation and the intrinsic frequency preferences of neurons. Trends Neurosci. 23:216–222. - PubMed
-
- Mainen, Z. F., and T. J. Sejnowski. 1996. Influence of dendritic structure on firing pattern in model neocortical neurons. Nature. 382:363–366. - PubMed
-
- Hille, B. 2001. Ionic Channels in Excitable Membranes. Sinauer Associates, Sunderland, MA.
-
- Marder, E., and A. A. Prinz. 2002. Modeling stability in neuron and network function: the role of activity in homeostasis. Bioessays. 24:1145–1154. - PubMed
-
- Stemmler, M., and C. Koch. 1999. How voltage-dependent conductances can adapt to maximize the information encoded by neuronal firing rate. Nat. Neurosci. 2:521–527. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

