Interaction of transported drugs with the lipid bilayer and P-glycoprotein through a solvation exchange mechanism
- PMID: 16565061
- PMCID: PMC1459527
- DOI: 10.1529/biophysj.105.077743
Interaction of transported drugs with the lipid bilayer and P-glycoprotein through a solvation exchange mechanism
Abstract
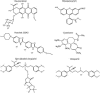
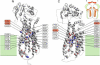
Broad substrate specificity of human P-glycoprotein (ABCB1) is an essential feature of multidrug resistance. Transport substrates of P-glycoprotein are mostly hydrophobic and many of them have net positive charge. These compounds partition into the membrane. Utilizing the energy of ATP hydrolysis, P-glycoprotein is thought to take up substrates from the cytoplasmic leaflet of the plasma membrane and to transport them to the outside of the cell. We examined this model by molecular dynamics simulation of the lipid bilayer, in the presence of transport substrates together with an atomic resolution structural model of P-glycoprotein. Taken together with previous electron paramagnetic resonance studies, the results suggest that most transported drugs are concentrated near the surface zone of the inner leaflet of the plasma membrane. Here the drugs can easily diffuse laterally into the drug-binding site of P-glycoprotein through an open cleft. It was concluded that the initial high-affinity drug-binding site was located in the interfacial surface area of P-glycoprotein in contact with the membrane interface. Based on these results and our recent kinetic studies, a "solvation exchange" drug transport mechanism of P-glycoprotein is discussed. A molecular basis for the improved colchicine transport efficiency by the much-studied colchicine-resistance G185V mutant human P-glycoprotein is also provided.
Figures



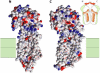

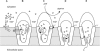
Comment in
-
Unraveling membrane-mediated substrate-transporter interactions.Biophys J. 2006 Jun 1;90(11):3825-6. doi: 10.1529/biophysj.106.082008. Epub 2006 Mar 24. Biophys J. 2006. PMID: 16565039 Free PMC article. No abstract available.
References
-
- Gottesman, M. M., and I. Pastan. 1993. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu. Rev. Biochem. 62:385–427. - PubMed
-
- Borst, P., and R. O. Elferink. 2002. Mammalian ABC transporters in health and disease. Annu. Rev. Biochem. 71:537–592. - PubMed
-
- Ambudkar, S. V., C. Kimchi-Sarfaty, Z. E. Sauna, and M. M. Gottesman. 2003. P-glycoprotein: from genomics to mechanism. Oncogene. 22:7468–7485. - PubMed
-
- Leslie, E. M., R. G. Deeley, and S. P. Cole. 2005. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol. Appl. Pharmacol. 204:216–237. - PubMed
-
- Senior, A. E., M. K. Al-Shawi, and I. L. Urbatsch. 1995. ATP hydrolysis by multidrug-resistance protein from Chinese hamster ovary cells. J. Bioenerg. Biomembr. 27:31–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

