Cytoplasmic gamma-actin contributes to a compensatory remodeling response in dystrophin-deficient muscle
- PMID: 16565216
- PMCID: PMC1459364
- DOI: 10.1073/pnas.0600980103
Cytoplasmic gamma-actin contributes to a compensatory remodeling response in dystrophin-deficient muscle
Abstract
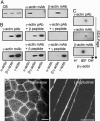
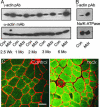
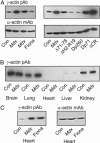
Dystrophin mechanically links the costameric cytoskeleton and sarcolemma, yet dystrophin-deficient muscle exhibits abnormalities in cell signaling, gene expression, and contractile function that are not clearly understood. We generated new antibodies specific for cytoplasmic gamma-actin and confirmed that gamma-actin most predominantly localized to the sarcolemma and in a faint reticular lattice within normal muscle cells. However, we observed that gamma-actin levels were increased 10-fold at the sarcolemma and within the cytoplasm of striated muscle cells from dystrophin-deficient mdx mice. Transgenic overexpression of the dystrophin homologue utrophin, or functional dystrophin constructs in mdx muscle, restored gamma-actin to normal levels, whereas gamma-actin remained elevated in mdx muscle expressing nonfunctional dystrophin constructs. We conclude that increased cytoplasmic gamma-actin in dystrophin-deficient muscle may be a compensatory response to fortify the weakened costameric lattice through recruitment of parallel mechanical linkages. However, the presence of excessive myoplasmic gamma-actin may also contribute to altered cell signaling or gene expression in dystrophin-deficient muscle.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


Similar articles
-
The dystrophin complex forms a mechanically strong link between the sarcolemma and costameric actin.J Cell Biol. 2000 Sep 4;150(5):1209-14. doi: 10.1083/jcb.150.5.1209. J Cell Biol. 2000. PMID: 10974007 Free PMC article.
-
Utrophin binds laterally along actin filaments and can couple costameric actin with sarcolemma when overexpressed in dystrophin-deficient muscle.Mol Biol Cell. 2002 May;13(5):1512-21. doi: 10.1091/mbc.01-09-0446. Mol Biol Cell. 2002. PMID: 12006649 Free PMC article.
-
Cytoplasmic gamma-actin expression in diverse animal models of muscular dystrophy.Neuromuscul Disord. 2007 Jul;17(7):569-74. doi: 10.1016/j.nmd.2007.03.004. Epub 2007 May 1. Neuromuscul Disord. 2007. PMID: 17475492 Free PMC article.
-
An attempt of gene therapy in Duchenne muscular dystrophy: overexpression of utrophin in transgenic mdx mice.Acta Neurol Belg. 2000 Sep;100(3):146-50. Acta Neurol Belg. 2000. PMID: 11098286 Review.
-
Dystrophin and the membrane skeleton.Curr Opin Cell Biol. 1993 Feb;5(1):82-7. doi: 10.1016/s0955-0674(05)80012-2. Curr Opin Cell Biol. 1993. PMID: 8448034 Review.
Cited by
-
Localizations of γ-Actins in Skin, Hair, Vibrissa, Arrector Pili Muscle and Other Hair Appendages of Developing Rats.Acta Histochem Cytochem. 2016 Apr 28;49(2):47-65. doi: 10.1267/ahc.15031. Epub 2016 Apr 9. Acta Histochem Cytochem. 2016. PMID: 27222613 Free PMC article.
-
Multi-isotope imaging mass spectrometry reveals slow protein turnover in hair-cell stereocilia.Nature. 2012 Jan 15;481(7382):520-4. doi: 10.1038/nature10745. Nature. 2012. PMID: 22246323 Free PMC article.
-
Variable cytoplasmic actin expression impacts the sensitivity of different dystrophin-deficient mdx skeletal muscles to eccentric contraction.FEBS J. 2019 Jul;286(13):2562-2576. doi: 10.1111/febs.14831. Epub 2019 Apr 11. FEBS J. 2019. PMID: 30942954 Free PMC article.
-
Tropomodulin 1 directly controls thin filament length in both wild-type and tropomodulin 4-deficient skeletal muscle.Development. 2015 Dec 15;142(24):4351-62. doi: 10.1242/dev.129171. Epub 2015 Nov 19. Development. 2015. PMID: 26586224 Free PMC article.
-
Actin in hair cells and hearing loss.Hear Res. 2012 Jun;288(1-2):89-99. doi: 10.1016/j.heares.2011.12.003. Epub 2011 Dec 13. Hear Res. 2012. PMID: 22200607 Free PMC article. Review.
References
-
- O’Brien K. F., Kunkel L. M. Mol. Genet. Metab. 2001;74:75–88. - PubMed
-
- Cohn R. D., Campbell K. P. Muscle Nerve. 2000;23:1456–1471. - PubMed
-
- Blake D. J., Weir A., Newey S. E., Davies K. E. Physiol. Rev. 2002;82:291–329. - PubMed
-
- Ervasti J. M. J. Biol. Chem. 2003;278:13591–13594. - PubMed
-
- Suzuki A., Yoshida M., Yamamoto H., Ozawa E. FEBS Lett. 1992;308:154–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

