Costs and benefits of priming for defense in Arabidopsis
- PMID: 16565218
- PMCID: PMC1459400
- DOI: 10.1073/pnas.0510213103
Costs and benefits of priming for defense in Arabidopsis
Abstract
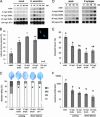
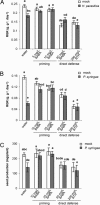
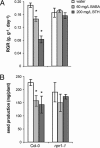
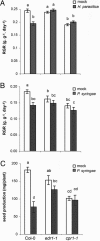
Induced resistance protects plants against a wide spectrum of diseases; however, it can also entail costs due to the allocation of resources or toxicity of defensive products. The cellular defense responses involved in induced resistance are either activated directly or primed for augmented expression upon pathogen attack. Priming for defense may combine the advantages of enhanced disease protection and low costs. In this study, we have compared the costs and benefits of priming to those of induced direct defense in Arabidopsis. In the absence of pathogen infection, chemical priming by low doses of beta-aminobutyric acid caused minor reductions in relative growth rate and had no effect on seed production, whereas induction of direct defense by high doses of beta-aminobutyric acid or benzothiadiazole strongly affected both fitness parameters. These costs were defense-related, because the salicylic acid-insensitive defense mutant npr1-1 remained unaffected by these treatments. Furthermore, the constitutive priming mutant edr1-1 displayed only slightly lower levels of fitness than wild-type plants and performed considerably better than the constitutively activated defense mutant cpr1-1. Hence, priming involves less fitness costs than induced direct defense. Upon infection by Pseudomonas syringae or Hyaloperonospora parasitica, priming conferred levels of disease protection that almost equaled the protection in benzothiadiazole-treated wild-type plants and cpr1 plants. Under these conditions, primed plants displayed significantly higher levels of fitness than noninduced plants and plants expressing chemically or cpr1-induced direct defense. Collectively, our results indicate that the benefits of priming-mediated resistance outweigh the costs in environments in which disease occurs.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

