Controlling methicillin-resistant Staphylococcus aureus: quantifying the effects of interventions and rapid diagnostic testing
- PMID: 16565219
- PMCID: PMC1459403
- DOI: 10.1073/pnas.0510077103
Controlling methicillin-resistant Staphylococcus aureus: quantifying the effects of interventions and rapid diagnostic testing
Abstract
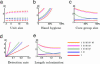
Control of nosocomial transmission of methicillin-resistant Staphylococcus aureus (MRSA) has been unsuccessful in most countries. Yet, some countries have maintained low endemic levels by implementing nationwide MRSA-specific infection control measures, such as "search & destroy" (S&D). These strategies, however, are not based on well designed studies, and their use in countries with high levels of endemicity is controversial. We present a stochastic three-hospital model and an analytical one-hospital model to quantify the effectiveness of different infection control measures and to predict the effects of rapid diagnostic testing (RDT) on isolation needs. Isolation of MRSA carriers identified by clinical cultures is insufficient to control MRSA. However, combined with proactive search (of high-risk patients on admission and/or contacts of index patients), it will maintain prevalence levels <1%. Concerted implementation of S&D in countries with high nosocomial endemicity reduces nosocomial prevalence to <1% within 6 years. Stepwise implementation of control measures can reduce isolation capacities needed. RDT can reduce isolation needs by >90% in low-endemic settings and by 20% in high-endemic settings. Surveillance of colonization and improved hand hygiene can markedly increase control efficacy. These findings strongly suggest that: (i) causality exists between S&D and low MRSA prevalence; (ii) isolating MRSA carriers identified by clinical cultures as a single measure is insufficient for control; (iii) a combined approach of isolation and screening confers efficacy; and (iv) MRSA-prevalence levels can be reduced to <1% in high-endemic settings by S&D or a stepwise approach to interventions. RDT can markedly enhance feasibility.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
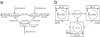
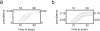
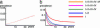


References
-
- Zetola N., Francis J. S., Nuermberger E. L., Bishai W.R. Lancet Infect. Dis. 2005;5:275–286. - PubMed
-
- Salgado C. D., Farr B. M., Calfee D. P. Clin. Infect. Dis. 2003;36:131–139. - PubMed
-
- Pastila S., Sammalkorpi K. T., Vuopio-Varkila J., Kontiainen S., Ristola M. A. J. Hosp. Infect. 2004;58:180–186. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

