The critical roles of serum/glucocorticoid-regulated kinase 3 (SGK3) in the hair follicle morphogenesis and homeostasis: the allelic difference provides novel insights into hair follicle biology
- PMID: 16565488
- PMCID: PMC1606558
- DOI: 10.2353/ajpath.2006.050507
The critical roles of serum/glucocorticoid-regulated kinase 3 (SGK3) in the hair follicle morphogenesis and homeostasis: the allelic difference provides novel insights into hair follicle biology
Abstract
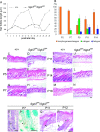
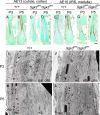
Mutation in the serum/glucocorticoid regulated kinase 3 (Sgk3, also known as Sgkl or Cisk) gene causes both defective hair follicle development and altered hair cycle in mice. We examined Sgk3-mutant YPC mice (YPC-Sgk3(ypc)/Sgk3(ypc)) and found expression of SGK3 protein with altered function. In the hair follicles of YPC mice, the aberrant differentiation and poor proliferation of hair matrix keratinocytes during the period of postnatal hair follicle development resulted in a complete lack of hair medulla and weak hair. Surprisingly, the length of postnatal hair follicle development and anagen term was shown to be dramatically shortened. Also, phosphorylation of GSK3beta at Ser9 and the nuclear accumulation of beta-catenin were reduced in the developing YPC hair follicle, suggesting that phosphorylation of GSK3beta and WNT-beta-catenin pathway takes part in the SGK3-dependent regulation of hair follicle development. Moreover, the above-mentioned features, especially the hair-cycling pattern, differ from those in other Sgk3-null mutant strains, suggesting that the various patterns of dysfunction in the SGK3 protein may result in phenotypic variation. Our results indicate that SGK3 is a very important and characteristic molecule that plays a critical role in both hair follicle morphogenesis and hair cycling.
Figures



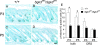


Similar articles
-
Akt2 and SGK3 are both determinants of postnatal hair follicle development.FASEB J. 2009 Sep;23(9):3193-202. doi: 10.1096/fj.08-123729. Epub 2009 May 11. FASEB J. 2009. PMID: 19433625 Free PMC article.
-
Targeted disruption of the protein kinase SGK3/CISK impairs postnatal hair follicle development.Mol Biol Cell. 2004 Sep;15(9):4278-88. doi: 10.1091/mbc.e04-01-0027. Epub 2004 Jul 7. Mol Biol Cell. 2004. PMID: 15240817 Free PMC article.
-
A mutation in the serum and glucocorticoid-inducible kinase-like kinase (Sgkl) gene is associated with defective hair growth in mice.DNA Res. 2004 Dec 31;11(6):371-9. doi: 10.1093/dnares/11.6.371. DNA Res. 2004. PMID: 15871460
-
Beauty is skin deep: the fascinating biology of the epidermis and its appendages.Harvey Lect. 1998-1999;94:47-77. Harvey Lect. 1998. PMID: 11070952 Review.
-
Mutant laboratory mice with abnormalities in hair follicle morphogenesis, cycling, and/or structure: an update.J Dermatol Sci. 2013 Jan;69(1):6-29. doi: 10.1016/j.jdermsci.2012.10.001. Epub 2012 Oct 16. J Dermatol Sci. 2013. PMID: 23165165 Review.
Cited by
-
Akt2 and SGK3 are both determinants of postnatal hair follicle development.FASEB J. 2009 Sep;23(9):3193-202. doi: 10.1096/fj.08-123729. Epub 2009 May 11. FASEB J. 2009. PMID: 19433625 Free PMC article.
-
Identification of selection signals by large-scale whole-genome resequencing of cashmere goats.Sci Rep. 2017 Nov 9;7(1):15142. doi: 10.1038/s41598-017-15516-0. Sci Rep. 2017. PMID: 29123196 Free PMC article.
-
Blunted IgE-mediated activation of mast cells in mice lacking the serum- and glucocorticoid-inducible kinase SGK3.Am J Physiol Cell Physiol. 2010 Nov;299(5):C1007-14. doi: 10.1152/ajpcell.00539.2009. Epub 2010 Aug 4. Am J Physiol Cell Physiol. 2010. PMID: 20686074 Free PMC article.
-
Regulation of gastric acid secretion by the serum and glucocorticoid inducible kinase isoform SGK3.J Gastroenterol. 2011 Mar;46(3):305-17. doi: 10.1007/s00535-010-0348-8. Epub 2010 Nov 27. J Gastroenterol. 2011. PMID: 21113728 Free PMC article.
References
-
- Fuchs E, Merrill BJ, Jamora C, DasGupta R. At the roots of a never-ending cycle. Dev Cell. 2001;1:13–25. - PubMed
-
- Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–494. - PubMed
-
- Niemann C, Watt FM. Designer skin: lineage commitment in postnatal epidermis. Trends Cell Biol. 2002;12:185–192. - PubMed
-
- Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341:491–497. - PubMed
-
- Muller-Rover S, Handjiski B, van der Veen C, Eichmuller S, Foitzik K, McKay IA, Stenn KS, Paus R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol. 2001;117:3–15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

