Kupffer cell-dependent hepatitis occurs during influenza infection
- PMID: 16565492
- PMCID: PMC1606556
- DOI: 10.2353/ajpath.2006.050875
Kupffer cell-dependent hepatitis occurs during influenza infection
Abstract
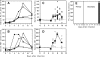
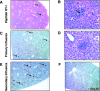
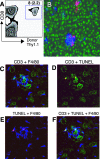
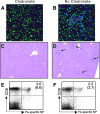
Respiratory infections, including influenza in humans, are often accompanied by a hepatitis that is usually mild and self-limiting. The mechanism of this kind of liver damage is not well understood. In the present study, we show that influenza-associated hepatitis occurs due to the formation of inflammatory foci that include apoptotic hepatocytes, antigen-specific CD8(+) T cells, and Kupffer cells. Serum aminotransaminase levels were elevated, and both the histological and serum enzyme markers of hepatitis were increased in secondary influenza infection, consistent with a primary role for antigen-specific T cells in the pathogenesis. No virus could be detected in the liver, making this a pure example of "collateral damage" of the liver. Notably, removal of the Kupffer cells prevented the hepatitis. Such hepatic collateral damage may be a general consequence of expanding CD8(+) T-cell populations during many extrahepatic viral infections, yielding important implications for liver pathobiology.
Figures


Comment in
-
Systemic viral infections and collateral damage in the liver.Am J Pathol. 2006 Apr;168(4):1057-9. doi: 10.2353/ajpath.2006.051296. Am J Pathol. 2006. PMID: 16565481 Free PMC article. No abstract available.
-
Ischemic hepatitis and collateral damage to the liver in severe viral respiratory tract infections.Am J Pathol. 2006 Sep;169(3):1100; author reply 1100. doi: 10.2353/ajpath.2006.060514. Am J Pathol. 2006. PMID: 16936282 Free PMC article. No abstract available.
References
-
- Wirth S. [“Accompanying hepatitis” in viral infections]. Dtsch Med Wochenschr. 1995;120:461. - PubMed
-
- Yuen KY, Chan PK, Peiris M, Tsang DN, Que TL, Shortridge KF, Cheung PT, To WK, Ho ET, Sung R, Cheng AF. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998;351:467–471. - PubMed
-
- Chan PK. Outbreak of avian influenza A(H5N1) virus infection in Hong Kong in 1997. Clin Infect Dis. 2002;34(Suppl 2):S58–S64. - PubMed
-
- Huang L, Soldevila G, Leeker M, Flavell R, Crispe IN. The liver eliminates T cells undergoing antigen-triggered apoptosis in vivo. Immunity. 1994;1:741–749. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

