Type I interferon production by tertiary lymphoid tissue developing in response to 2,6,10,14-tetramethyl-pentadecane (pristane)
- PMID: 16565497
- PMCID: PMC1606560
- DOI: 10.2353/ajpath.2006.050125
Type I interferon production by tertiary lymphoid tissue developing in response to 2,6,10,14-tetramethyl-pentadecane (pristane)
Abstract
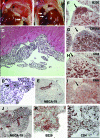
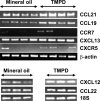
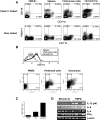
Lymphoid neogenesis is associated with antibody-mediated autoimmune diseases such as Sjogren's syndrome and rheumatoid arthritis. Although systemic lupus erythematosus is the prototypical B-cell-mediated autoimmune disease, the role of lymphoid neogenesis in its pathogenesis is unknown. Intraperitoneal injection of 2,6,10,14-tetramethyl-pentadecane (TMPD, pristane) or mineral oil causes lipogranuloma formation in mice, but only TMPD-treated mice develop lupus. We report that lipogranulomas are a form of lymphoid neogenesis. Immunoperoxidase staining of lipogranulomas revealed B cells, CD4(+) T cells, and dendritic cells and in some cases organization into T- and B-cell zones. Lipogranulomas also expressed the lymphoid chemokines CCL21, CCL19, CXCL13, CXCL12, and CCL22. Expression of the type I interferon (IFN-I)-inducible genes Mx1, IRF7, IP-10, and ISG-15 was greatly increased in TMPD- versus mineral oil-induced lipogranulomas. Dendritic cells from TMPD lipogranulomas underwent activation/maturation with high CD86 and interleukin-12 expression. Magnetic bead depletion of dendritic cells markedly diminished IFN-inducible gene (Mx1) expression. We conclude that TMPD-induced lupus is associated with the formation of ectopic lymphoid tissue containing activated dendritic cells producing IFN-I and interleukin-12. In view of the increased IFN-I production in systemic lupus erythematosus, these studies suggest that IFN-I from ectopic lymphoid tissue could play a role in the pathogenesis of experimental lupus in mice.
Figures



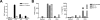
Similar articles
-
B cell proliferation, somatic hypermutation, class switch recombination, and autoantibody production in ectopic lymphoid tissue in murine lupus.J Immunol. 2009 Apr 1;182(7):4226-36. doi: 10.4049/jimmunol.0800771. J Immunol. 2009. PMID: 19299721 Free PMC article.
-
Pleiotropic IFN-dependent and -independent effects of IRF5 on the pathogenesis of experimental lupus.J Immunol. 2012 Apr 15;188(8):4113-21. doi: 10.4049/jimmunol.1103113. Epub 2012 Mar 14. J Immunol. 2012. PMID: 22422888 Free PMC article.
-
Role of Lgals9 Deficiency in Attenuating Nephritis and Arthritis in BALB/c Mice in a Pristane-Induced Lupus Model.Arthritis Rheumatol. 2018 Jul;70(7):1089-1101. doi: 10.1002/art.40467. Epub 2018 May 24. Arthritis Rheumatol. 2018. PMID: 29481735
-
Induction of autoimmunity by pristane and other naturally occurring hydrocarbons.Trends Immunol. 2009 Sep;30(9):455-64. doi: 10.1016/j.it.2009.06.003. Epub 2009 Aug 19. Trends Immunol. 2009. PMID: 19699150 Free PMC article. Review.
-
Ectopic lymphoid neogenesis and lymphoid chemokines in Sjogren's syndrome: at the interplay between chronic inflammation, autoimmunity and lymphomagenesis.Curr Pharm Biotechnol. 2012 Aug;13(10):1989-96. doi: 10.2174/138920112802273209. Curr Pharm Biotechnol. 2012. PMID: 22208651 Review.
Cited by
-
High-dimensional analysis reveals a pathogenic role of inflammatory monocytes in experimental diffuse alveolar hemorrhage.JCI Insight. 2019 Aug 8;4(15):e129703. doi: 10.1172/jci.insight.129703. eCollection 2019 Aug 8. JCI Insight. 2019. PMID: 31391335 Free PMC article.
-
Mouse models of lupus: what they tell us and what they don't.Lupus Sci Med. 2018 Jan 21;5(1):e000199. doi: 10.1136/lupus-2016-000199. eCollection 2018. Lupus Sci Med. 2018. PMID: 29387435 Free PMC article.
-
A fully human monoclonal antibody with novel binding epitope and excellent neutralizing activity to multiple human IFN-α subtypes: A candidate therapy for systemic lupus erythematosus.MAbs. 2015;7(5):969-80. doi: 10.1080/19420862.2015.1055443. MAbs. 2015. PMID: 26048268 Free PMC article.
-
Inflammatory monocyte-derived dendritic cells mediate autoimmunity in murine model of systemic lupus erythematosus.J Transl Autoimmun. 2020 Jul 15;3:100060. doi: 10.1016/j.jtauto.2020.100060. eCollection 2020. J Transl Autoimmun. 2020. PMID: 32743540 Free PMC article.
-
Differential Responsiveness of Monocyte and Macrophage Subsets to Interferon.Arthritis Rheumatol. 2020 Jan;72(1):100-113. doi: 10.1002/art.41072. Epub 2019 Dec 10. Arthritis Rheumatol. 2020. PMID: 31390156 Free PMC article.
References
-
- Luther SA, Lopez T, Bai W, Hanahan D, Cyster JG. BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity. 2000;12:471–481. - PubMed
-
- Yoneyama H, Matsuno K, Zhang Y, Murai M, Itakura M, Ishikawa S, Hasegawa G, Naito M, Asakura H, Matsushima K. Regulation by chemokines of circulating dendritic cell precursors, and the formation of portal tract-associated lymphoid tissue, in a granulomatous liver disease. J Exp Med. 2001;193:35–49. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

