Deficiency of C4 from donor or recipient mouse fails to prevent renal allograft rejection
- PMID: 16565498
- PMCID: PMC1606553
- DOI: 10.2353/ajpath.2006.050360
Deficiency of C4 from donor or recipient mouse fails to prevent renal allograft rejection
Abstract
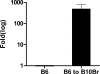
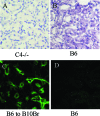
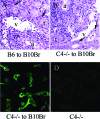
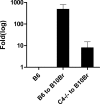
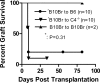
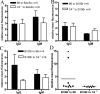
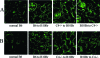
Complement effector products generated in the transplanted kidney are known to mediate transplant rejection, but which of the three main activation pathways of complement trigger this response is unclear. Here we assessed the role of the classical and lectin pathways by studying the common component C4 in mouse kidney transplant rejection. We transplanted wild-type or C4-null H-2(b) donor kidneys into H-2(k) or H-2(d) recipients, or vice-versa, to assess the roles of donor kidney and recipient expression of complement. Intragraft C4 gene expression rose substantially during rejection. However, we found no significant association between graft acceptance and the presence of C4 in either the donor kidney or recipient mouse. At the time of rejection, we found no significant differences in alloantibody response in the different groups. Tubular deposition of C3 to C9 occurred regardless of the absence or presence of C4 in either the donor or recipient mouse, indicating that C4 was dispensable for complement activation at this site. These data suggest that complement activation and renal allograft rejection are independent of the classical and lectin pathways in these models, implying that in the absence of these pathways the alternative pathway is the main trigger for complement-mediated rejection.
Figures




References
-
- Sacks SH, Chowdhury P, Zhou W. Role of the complement system in rejection. Curr Opin Immunol. 2003;15:487–492. - PubMed
-
- Baldwin WM, III, Larsen CP, Fairchild RL. Innate immune responses to transplants: a significant variable with cadaver donors. Immunity. 2001;14:369–376. - PubMed
-
- Korb LC, Ahearn JM. C1q binds directly and specifically to surface blebs of apoptotic human keratinocytes: complement deficiency and systemic lupus erythematosus revisited. J Immunol. 1997;158:4525–4528. - PubMed
-
- Gasque P. Complement: a unique innate immune sensor for danger signals. Mol Immunol. 2004;41:1089–1098. - PubMed
-
- Fujita T. Evolution of the lectin-complement pathway and its role in innate immunity. Nat Rev Immunol. 2002;2:346–353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

