Peroxisomal multifunctional protein-2 deficiency causes motor deficits and glial lesions in the adult central nervous system
- PMID: 16565505
- PMCID: PMC1606565
- DOI: 10.2353/ajpath.2006.041220
Peroxisomal multifunctional protein-2 deficiency causes motor deficits and glial lesions in the adult central nervous system
Abstract
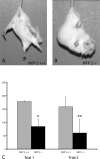
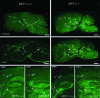
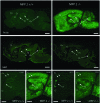
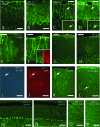
In humans, mutations inactivating multifunctional protein-2 (MFP-2), and thus peroxisomal beta-oxidation, cause neuronal heterotopia and demyelination, which is clinically reflected by hypotonia, seizures, and death within the first year of life. In contrast, our recently generated MFP-2-deficient mice did not show neurodevelopmental abnormalities but exhibited aberrations in bile acid metabolism and one of three of them died early postnatally. In the postweaning period, all survivors developed progressive motor deficits, including abnormal cramping reflexes of the limbs and loss of mobility, with death at 6 months. Motor impairment was not accompanied by lesions of peripheral nerves or muscles. However, in the central nervous system MFP-2-deficient mice overexpressed catalase in glial cells, accumulated lipids in ependymal cells and in the molecular layer of the cerebellum, exhibited severe astrogliosis and reactive microglia predominantly within the gray matter of the brain and the spinal cord, whereas synaptic and myelin markers were not affected. This culminated in degenerative changes of astroglia cells but not in overt neuronal lesions. Neither the motor deficits nor the brain lesions were aggravated by increasing the branched-chain fatty acid concentration through dietary supplementation. These data indicate that MFP-2 deficiency in mice causes a neurological phenotype in adulthood that is manifested primarily by astroglial damage.
Figures


References
-
- Van Veldhoven PP, Casteels M, Mannaerts GP, Baes M. Further insights into peroxisomal lipid breakdown via α- and β-oxidation. Biochem Soc Trans. 2001;29:292–298. - PubMed
-
- Wanders RJA, Vreken P, Ferdinandusse S, Jansen GA, Waterham HR, Van Roermund CWT, van Grunsven EG. Peroxisomal fatty acid α- and β-oxidation in humans: enzymology, peroxisomal metabolite transporters and peroxisomal diseases. Biochem Soc Trans. 2001;29:250–267. - PubMed
-
- Sprecher H. Metabolism of highly unsaturated n-3 and n-6 fatty acids. Biochim Biophys Acta. 2000;1486:219–231. - PubMed
-
- Ferdinandusse S, Meissner T, Wanders RJA, Mayatepek E. Identification of the peroxisomal β-oxidation enzymes involved in the degradation of leukotrienes. Biochem Biophys Res Commun. 2002;293:269–273. - PubMed
-
- Powers JM. The pathology of peroxisomal disorders with pathogenetic considerations. J Neuropathol Exp Neurol. 1995;54:710–719. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

