A novel aspartic proteinase-like gene expressed in stratified epithelia and squamous cell carcinoma of the skin
- PMID: 16565508
- PMCID: PMC1606566
- DOI: 10.2353/ajpath.2006.050871
A novel aspartic proteinase-like gene expressed in stratified epithelia and squamous cell carcinoma of the skin
Abstract
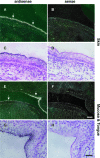
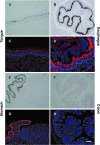
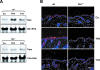
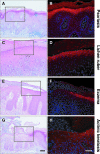
Homeostasis of stratified epithelia, such as the epidermis of the skin, is a sophisticated process that represents a tightly controlled balance between proliferation and differentiation. Alterations of this balance are associated with common human diseases including cancer. Here, we report the cloning of a novel cDNA sequence, from mouse back skin, that is induced by the phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA) and codes for a hitherto unknown aspartic proteinase-like protein (Taps). Taps represents a potential AP-1 target gene because TPA-induced expression in epidermal keratinocytes critically depends on c-Fos, and co-treatment with dexamethasone, a potent inhibitor of AP-1-mediated gene regulation, resulted in impaired activation of Taps expression. Taps mRNA and protein are restricted to stratified epithelia in mouse embryos and adult tissues, implicating a crucial role for this aspartic proteinase-like gene in differentiation and homeostasis of multilayered epithelia. During chemically induced carcinogenesis, transient elevation of Taps mRNA and protein levels was detected in benign skin tumors. However, its expression is negatively associated with dedifferentiation and malignant progression in squamous cell carcinomas of the skin. Similar expression was observed in squamous skin tumors of patients, suggesting that detection of Taps levels represents a novel strategy to discriminate the progression state of squamous skin cancers.
Figures




References
-
- Presland RB, Jurevic RJ. Making sense of the epithelial barrier: what molecular biology and genetics tell us about the functions of oral mucosal and epidermal tissues. J Dent Educ. 2002;66:564–574. - PubMed
-
- Fuchs E, Raghavan S. Getting under the skin of epidermal morphogenesis. Nat Rev Genet. 2002;3:199–209. - PubMed
-
- Koster MI, Roop DR. Genetic pathways required for epidermal morphogenesis. Eur J Cell Biol. 2004;83:625–629. - PubMed
-
- Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol. 2005;6:328–340. - PubMed
-
- Zeeuwen PL. Epidermal differentiation: the role of proteases and their inhibitors. Eur J Cell Biol. 2004;83:761–773. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

