Prolactin potentiates transforming growth factor alpha induction of mammary neoplasia in transgenic mice
- PMID: 16565509
- PMCID: PMC1606572
- DOI: 10.2353/ajpath.2006.050861
Prolactin potentiates transforming growth factor alpha induction of mammary neoplasia in transgenic mice
Abstract
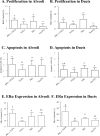
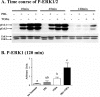
Prolactin influences mammary development and carcinogenesis through endocrine and autocrine/paracrine mechanisms. In virgin female mice, pro-lactin overexpression under control of a mammary selective nonhormonally responsive promoter, neu-related lipocalin, results in estrogen receptor alpha (ERalpha)-positive and ERalpha-negative adenocarcinomas. However, disease in vivo occurs in the context of dysregulation of multiple pathways. In this study, we investigated the ability of prolactin to modulate carcinogenesis when co-expressed with the potent oncogene transforming growth factor alpha (TGFalpha) in bitransgenic mice. Prolactin and TGFalpha cooperated to reduce dramatically the latency of mammary macrocyst development, the principal lesion type induced by TGFalpha. In combination, prolactin and TGFalpha also increased the incidence and reduced the latency of other preneoplastic lesions and increased cellular turnover in structurally normal alveoli and ducts compared with single transgenic females. Bitransgenic glands contained higher levels of phosphorylated ERK1/2 compared with single TGFalpha transgenic glands, suggesting that this kinase may be a point of signaling crosstalk. Furthermore, transgenic prolactin also reversed the decrease in ERalpha induced by neu-related lipocalin-TGFalpha. Our findings demonstrate that locally produced prolactin can strikingly potentiate the carcinogenic actions of another oncogene and modify ovarian hormone responsiveness, suggesting that prolactin signaling may be a potential therapeutic target.
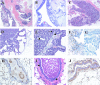
Figures

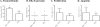
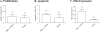

References
-
- Reynolds C, Montone KT, Powell CM, Tomaszewski JE, Clevenger CV. Expression of prolactin and its receptor in human breast carcinoma. Endocrinology. 1997;138:5555–5560. - PubMed
-
- Mertani HC, Garcia-Caballero T, Lambert A, Gérard F, Palayer C, Boutin JM, Vonderhaar BK, Waters MJ, Lobie PE, Morel G. Cellular expression of growth hormone and prolactin receptors in human breast disorders. Int J Cancer. 1998;79:202–211. - PubMed
-
- Touraine P, Martini JF, Zafrani B, Durand JC, Labaille F, Malet C, Nicolas A, Trivin C, Postel-Vinay MC, Kuttenn F, Kelly PA. Increased expression of prolactin receptor gene assessed by quantitative polymerase chain reaction in human breast tumors versus normal breast tissues. J Clin Endocrinol Metab. 1998;83:667–674. - PubMed
-
- Vonderhaar BK. Prolactin in human breast cancer development. Ethier SP, editor. Humana Press; Totawa, NJ: Endocrine Oncology. 2000:pp 101–120.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

