Proteomic profiling of metalloprotease activities with cocktails of active-site probes
- PMID: 16565715
- PMCID: PMC1538544
- DOI: 10.1038/nchembio781
Proteomic profiling of metalloprotease activities with cocktails of active-site probes
Abstract
Metalloproteases are a large, diverse class of enzymes involved in many physiological and disease processes. Metalloproteases are regulated by post-translational mechanisms that diminish the effectiveness of conventional genomic and proteomic methods for their functional characterization. Chemical probes directed at active sites offer a potential way to measure metalloprotease activities in biological systems; however, large variations in structure limit the scope of any single small-molecule probe aimed at profiling this enzyme class. Here, we address this problem by creating a library of metalloprotease-directed probes that show complementary target selectivity. These probes were applied as a 'cocktail' to proteomes and their labeling profiles were analyzed collectively using an advanced liquid chromatography-mass spectrometry platform. More than 20 metalloproteases were identified, including members from nearly all of the major branches of this enzyme class. These findings suggest that chemical proteomic methods can serve as a universal strategy to profile the activity of the metalloprotease superfamily in complex biological systems.
Figures



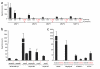
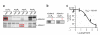
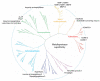
Comment in
-
Metalloproteases see the light.Nat Chem Biol. 2006 May;2(5):229-30. doi: 10.1038/nchembio0506-229. Nat Chem Biol. 2006. PMID: 16619018 No abstract available.
References
-
- Gerlt JA, Babbitt PC. Divergent evolution of enzymatic function: mechanistically diverse superfamilies and functionally distinct suprafamilies. Annu. Rev. Biochem. 2001;70:209–246. - PubMed
-
- Saghatelian A, Cravatt BF. Assignment of protein function in the postgenomic era. Nat. Chem. Biol. 2005;1:130–142. - PubMed
-
- Kobe B, Kemp BE. Active site-directed protein regulation. Nature. 1999;402:373–376. - PubMed
-
- Jessani N, Cravatt BF. The development and application of methods for activity-based protein profiling. Curr. Opin. Chem. Biol. 2004;8:54–59. - PubMed
Publication types
MeSH terms
Substances
Associated data
- PubChem-Substance/10318689
- PubChem-Substance/10318690
- PubChem-Substance/10318691
- PubChem-Substance/10318692
- PubChem-Substance/10318693
- PubChem-Substance/10318694
- PubChem-Substance/10318695
- PubChem-Substance/10318696
- PubChem-Substance/10318697
- PubChem-Substance/10318698
- PubChem-Substance/10318699
- PubChem-Substance/10318700
- PubChem-Substance/10318701
- PubChem-Substance/10318702
- PubChem-Substance/10318703
- PubChem-Substance/10318704
- PubChem-Substance/10318705
- PubChem-Substance/10318706
- PubChem-Substance/10318707
- PubChem-Substance/10318708
- PubChem-Substance/10318709
- PubChem-Substance/10318710
- PubChem-Substance/10318711
- PubChem-Substance/10318712
- PubChem-Substance/10318713
- PubChem-Substance/10318714
- PubChem-Substance/10318715
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

