Projections of the second cervical dorsal root ganglion to the cochlear nucleus in rats
- PMID: 16566003
- PMCID: PMC2736115
- DOI: 10.1002/cne.20917
Projections of the second cervical dorsal root ganglion to the cochlear nucleus in rats
Abstract
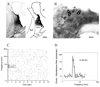
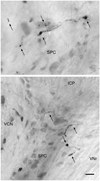
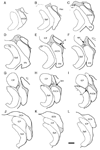
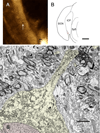
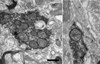
Physiological, anatomical, and clinical data have demonstrated interactions between somatosensory and auditory brainstem structures. Spinal nerve projections influence auditory responses, although the nature of the pathway(s) is not known. To address this issue, we injected biotinylated dextran amine into the cochlear nucleus or dorsal root ganglion (DRG) at the second cervical segment (C2). Cochlear nucleus injections retrogradely labeled small ganglion cells in C2 DRG. C2 DRG injections produced anterograde labeling in the external cuneate nucleus, cuneate nucleus, nucleus X, central cervical nucleus, dorsal horn of upper cervical spinal segments, and cochlear nucleus. The terminal field in the cochlear nucleus was concentrated in the subpeduncular corner and lamina of the granule cell domain, where endings of various size and shapes appeared. Examination under an electron microscope revealed that the C2 DRG terminals contained numerous round synaptic vesicles and formed asymmetric synapses, implying depolarizing influences on the target cell. Labeled endings synapsed with the stalk of the primary dendrite of unipolar brush cells, distal dendrites of presumptive granule cells, and endings containing pleomorphic synaptic vesicles. These primary somatosensory projections contribute to circuits that are hypothesized to mediate integrative functions of hearing.
Copyright 2006 Wiley-Liss, Inc.
Figures




References
-
- Abel MD, Levine RA. Muscle contractions and auditory perception in tinnitus patients and nonclinical subjects. Cranio. 2004;22:181–191. - PubMed
-
- Abrahams VC, Richmond FJ, Keane J. Projections from C2 and C3 nerves supplying muscles and skin of the cat neck: a study using transganglionic transport of horseradish peroxidase. J Comp Neurol. 1984;230:142–154. - PubMed
-
- Bae YC, Park KS, Bae JY, Paik SK, Ahn DK, Moritani M, Yoshida A, Shigenaga Y. GABA and glycine in synaptic microcircuits associated with physiologically characterized primary afferents of cat trigeminal principal nucleus. Exp Brain Res. 2005;162:449–457. - PubMed
-
- Baxter DA, Bittner GD. Intracellular recordings from crustacean motor axons during presynaptic inhibition. Brain Res. 1981;233:422–428. - PubMed
-
- Chen K, Waller HJ, Godfrey TG, Godfrey DA. Glutamergic transmission of neuronal responses to carbachol in rat dorsal cochlear nucleus slices. Neurosci. 1999;90:1043–1049. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

