Immunolocalization of the Ca2+-activated K+ channel Slo1 in axons and nerve terminals of mammalian brain and cultured neurons
- PMID: 16566008
- PMCID: PMC2605666
- DOI: 10.1002/cne.20931
Immunolocalization of the Ca2+-activated K+ channel Slo1 in axons and nerve terminals of mammalian brain and cultured neurons
Abstract
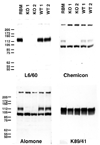
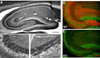
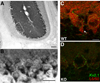
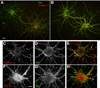
Ca(2+)-activated voltage-dependent K(+) channels (Slo1, KCa1.1, Maxi-K, or BK channel) play a crucial role in controlling neuronal signaling by coupling channel activity to both membrane depolarization and intracellular Ca(2+) signaling. In mammalian brain, immunolabeling experiments have shown staining for Slo1 channels predominantly localized to axons and presynaptic terminals of neurons. We have developed anti-Slo1 mouse monoclonal antibodies that have been extensively characterized for specificity of staining against recombinant Slo1 in heterologous cells, and native Slo1 in mammalian brain, and definitively by the lack of detectable immunoreactivity against brain samples from Slo1 knockout mice. Here we provide precise immunolocalization of Slo1 in rat brain with one of these monoclonal antibodies and show that Slo1 is accumulated in axons and synaptic terminal zones associated with glutamatergic synapses in hippocampus and GABAergic synapses in cerebellum. By using cultured hippocampal pyramidal neurons as a model system, we show that heterologously expressed Slo1 is initially targeted to the axonal surface membrane, and with further development in culture, become localized in presynaptic terminals. These studies provide new insights into the polarized localization of Slo1 channels in mammalian central neurons and provide further evidence for a key role in regulating neurotransmitter release in glutamatergic and GABAergic terminals.
Copyright 2006 Wiley-Liss, Inc.
Figures






References
-
- Ango F, di Cristo G, Higashiyama H, Bennett V, Wu P, Huang ZJ. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell. 2004;119:257–272. - PubMed
-
- Antonucci DE, Lim ST, Vassanelli S, Trimmer JS. Dynamic localization and clustering of dendritic Kv2.1 voltage-dependent potassium channels in developing hippocampal neurons. Neuroscience. 2001;108:69–81. - PubMed
-
- Arnold DB, Clapham DE. Molecular determinants for subcellular localization of PSD-95 with an interacting K+ channel. Neuron. 1999;23:149–157. - PubMed
-
- Banker GA, Cowan WM. Rat hippocampal neurons in dispersed cell culture. Brain Research. 1977;126:397–425. - PubMed
-
- Bekele-Arcuri Z, Matos MF, Manganas L, Strassle BW, Monaghan MM, Rhodes KJ, Trimmer JS. Generation and characterization of subtype-specific monoclonal antibodies to K+ channel alpha- and beta-subunit polypeptides. Neuropharmacology. 1996;35:851–865. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

