Aberrant metabolites in mouse models of congenital blinding diseases: formation and storage of retinyl esters
- PMID: 16566595
- PMCID: PMC1560103
- DOI: 10.1021/bi052382x
Aberrant metabolites in mouse models of congenital blinding diseases: formation and storage of retinyl esters
Abstract
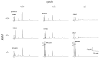
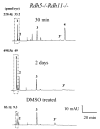
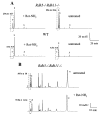
Regeneration of the visual chromophore, 11-cis-retinal, is a critical step in restoring photoreceptors to their dark-adapted conditions. This regeneration process, called the retinoid cycle, takes place in the photoreceptor outer segments and the retinal pigment epithelium (RPE). Disabling mutations in nearly all of the retinoid cycle genes are linked to human conditions that cause congenital or progressive defects in vision. Several mouse models with disrupted genes related to this cycle contain abnormal fatty acid retinyl ester levels in the RPE. To investigate the mechanisms of retinyl ester accumulation, we generated single or double knockout mice lacking retinoid cycle genes. All-trans-retinyl esters accumulated in mice lacking RPE65, but they are reduced in double knockout mice also lacking opsin, suggesting a connection between visual pigment regeneration and the retinoid cycle. Only Rdh5-deficient mice accumulate cis-retinyl esters, regardless of the simultaneous disruption of RPE65, opsin, and prRDH. 13-cis-Retinoids are produced at higher levels when the flow of retinoid through the cycle was increased, and these esters are stored in specific structures called retinosomes. Most importantly, retinylamine, a specific and effective inhibitor of the 11-cis-retinol formation, also inhibits the production of 13-cis-retinyl esters. The data presented here support the idea that 13-cis-retinyl esters are formed through an aberrant enzymatic isomerization process.
Figures


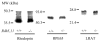


Similar articles
-
Retinyl esters are the substrate for isomerohydrolase.Biochemistry. 2003 Feb 25;42(7):2229-38. doi: 10.1021/bi026911y. Biochemistry. 2003. PMID: 12590612
-
Acute radiolabeling of retinoids in eye tissues of normal and rpe65-deficient mice.Invest Ophthalmol Vis Sci. 2003 Apr;44(4):1435-46. doi: 10.1167/iovs.02-0679. Invest Ophthalmol Vis Sci. 2003. PMID: 12657577
-
Rpe65 is a retinyl ester binding protein that presents insoluble substrate to the isomerase in retinal pigment epithelial cells.J Biol Chem. 2004 Jan 2;279(1):635-43. doi: 10.1074/jbc.M310042200. Epub 2003 Oct 7. J Biol Chem. 2004. PMID: 14532273
-
Key enzymes of the retinoid (visual) cycle in vertebrate retina.Biochim Biophys Acta. 2012 Jan;1821(1):137-51. doi: 10.1016/j.bbalip.2011.03.005. Epub 2011 Apr 5. Biochim Biophys Acta. 2012. PMID: 21447403 Free PMC article. Review.
-
Vitamin A derivatives as treatment options for retinal degenerative diseases.Nutrients. 2013 Jul 12;5(7):2646-66. doi: 10.3390/nu5072646. Nutrients. 2013. PMID: 23857173 Free PMC article. Review.
Cited by
-
An in silico toolbox for the prediction of the potential pathogenic effects of missense mutations in the dimeric region of hRPE65.J Enzyme Inhib Med Chem. 2023 Dec;38(1):2162047. doi: 10.1080/14756366.2022.2162047. J Enzyme Inhib Med Chem. 2023. PMID: 36629452 Free PMC article.
-
A novel cone visual cycle in the cone-dominated retina.Exp Eye Res. 2007 Aug;85(2):175-84. doi: 10.1016/j.exer.2007.05.003. Epub 2007 May 24. Exp Eye Res. 2007. PMID: 17618621 Free PMC article. Review.
-
A microparticle/hydrogel combination drug-delivery system for sustained release of retinoids.Invest Ophthalmol Vis Sci. 2012 Sep 19;53(10):6314-23. doi: 10.1167/iovs.12-10279. Invest Ophthalmol Vis Sci. 2012. PMID: 22918645 Free PMC article.
-
Effects of potent inhibitors of the retinoid cycle on visual function and photoreceptor protection from light damage in mice.Mol Pharmacol. 2006 Oct;70(4):1220-9. doi: 10.1124/mol.106.026823. Epub 2006 Jul 12. Mol Pharmacol. 2006. PMID: 16837623 Free PMC article.
-
Retinoid dynamics in vision: from visual cycle biology to retina disease treatments.Pharmacol Ther. 2025 Jun 21;273:108902. doi: 10.1016/j.pharmthera.2025.108902. Online ahead of print. Pharmacol Ther. 2025. PMID: 40550364 Free PMC article. Review.
References
-
- Ridge KD, Abdulaev NG, Sousa M, Palczewski K. Phototransduction: crystal clear. Trends Biochem Sci. 2003;28:479–487. - PubMed
-
- Okada T, Ernst OP, Palczewski K, Hofmann KP. Activation of rhodopsin: new insights from structural and biochemical studies. Trends Biochem Sci. 2001;26:318–324. - PubMed
-
- Rando, R. R. (1991) Membrane phospholipids as an energy source in the operation of the visual cycle, Biochemistry 30, 595–602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

