Secondary memory CD8+ T cells are more protective but slower to acquire a central-memory phenotype
- PMID: 16567385
- PMCID: PMC2118270
- DOI: 10.1084/jem.20052237
Secondary memory CD8+ T cells are more protective but slower to acquire a central-memory phenotype
Abstract
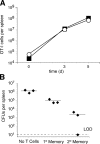
The formation of memory CD8 T cells is an important goal of vaccination. However, although widespread use of booster immunizations in humans generates secondary and tertiary CD8 T cell memory, experimental data are limited to primary CD8 T cell memory. Here, we show that, compared with primary memory CD8 T cells, secondary memory CD8 T cells exhibit substantially delayed conversion to a central-memory phenotype, as determined by CD62L expression and interleukin (IL)-2 production. This delayed conversion to a central-memory phenotype correlates with reduced basal proliferation and responsiveness to IL-15, although in vitro coculture with a high concentration of IL-15 is capable of inducing proliferation and CD62L upregulation. Functionally, secondary memory CD8 T cells are more protective in vivo on a per cell basis, and this may be explained by sustained lytic ability. Additionally, secondary memory CD8 T cells are more permissive than primary memory CD8 T cells for new T cell priming in lymph nodes, possibly suggesting a mechanism of replacement for memory T cells. Thus, primary and secondary memory CD8 T cells are functionally distinct, and the number of encounters with antigen influences memory CD8 T cell function.
Figures





Similar articles
-
Unidirectional development of CD8+ central memory T cells into protective Listeria-specific effector memory T cells.Eur J Immunol. 2006 Jun;36(6):1453-64. doi: 10.1002/eji.200635874. Eur J Immunol. 2006. PMID: 16637009
-
Reducing the stimulation of CD8+ T cells during infection with intracellular bacteria promotes differentiation primarily into a central (CD62LhighCD44high) subset.J Immunol. 2005 May 1;174(9):5341-50. doi: 10.4049/jimmunol.174.9.5341. J Immunol. 2005. PMID: 15843531
-
Simultaneous assessment of antigen-stimulated cytokine production and memory subset composition of memory CD8 T cells.J Immunol Methods. 2006 Jun 30;313(1-2):161-8. doi: 10.1016/j.jim.2006.04.005. Epub 2006 May 26. J Immunol Methods. 2006. PMID: 16762359
-
Memory CD8+ T cell differentiation.Ann N Y Acad Sci. 2010 Jan;1183:251-66. doi: 10.1111/j.1749-6632.2009.05126.x. Ann N Y Acad Sci. 2010. PMID: 20146720 Free PMC article. Review.
-
Defining Memory CD8 T Cell.Front Immunol. 2018 Nov 20;9:2692. doi: 10.3389/fimmu.2018.02692. eCollection 2018. Front Immunol. 2018. PMID: 30515169 Free PMC article. Review.
Cited by
-
Cooperativity between CD8+ T cells, non-neutralizing antibodies, and alveolar macrophages is important for heterosubtypic influenza virus immunity.PLoS Pathog. 2013 Mar;9(3):e1003207. doi: 10.1371/journal.ppat.1003207. Epub 2013 Mar 14. PLoS Pathog. 2013. PMID: 23516357 Free PMC article.
-
Viral vector vaccines make memory T cells against malaria.Immunology. 2007 Jun;121(2):158-65. doi: 10.1111/j.1365-2567.2006.02552.x. Immunology. 2007. PMID: 17462077 Free PMC article. Review.
-
Adenoviral vectors persist in vivo and maintain activated CD8+ T cells: implications for their use as vaccines.Blood. 2007 Sep 15;110(6):1916-23. doi: 10.1182/blood-2007-02-062117. Epub 2007 May 17. Blood. 2007. PMID: 17510320 Free PMC article.
-
Ubiquitin Specific Protease 1 Expression and Function in T Cell Immunity.J Immunol. 2021 Sep 1;207(5):1377-1387. doi: 10.4049/jimmunol.2100303. Epub 2021 Aug 11. J Immunol. 2021. PMID: 34380645 Free PMC article.
-
Different patterns of expansion, contraction and memory differentiation of HIV-1 Gag-specific CD8 T cells elicited by adenovirus type 5 and modified vaccinia Ankara vaccines.Vaccine. 2011 Jul 26;29(33):5399-406. doi: 10.1016/j.vaccine.2011.05.083. Epub 2011 Jun 7. Vaccine. 2011. PMID: 21651938 Free PMC article.
References
-
- Sprent, J., and C.D. Surh. 2002. T cell memory. Annu. Rev. Immunol. 20:551–579. - PubMed
-
- Woodland, D.L. 2004. Jump-starting the immune system: prime-boosting comes of antigene. Trends Immunol. 25:98–104. - PubMed
-
- Masopust, D., V. Vezys, A.L. Marzo, and L. Lefrancois. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 291:2413–2417. - PubMed
-
- Harty, J.T., and V.P. Badovinac. 2002. Influence of effector molecules on the CD8(+) T cell response to infection. Curr. Opin. Immunol. 14:360–365. - PubMed
-
- Harty, J.T., A.R. Tvinnereim, and D.W. White. 2000. CD8+ T cell effector mechanisms in resistance to infection. Annu. Rev. Immunol. 18:275–308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

