A unique requirement for the leukotriene B4 receptor BLT1 for neutrophil recruitment in inflammatory arthritis
- PMID: 16567386
- PMCID: PMC2118298
- DOI: 10.1084/jem.20052349
A unique requirement for the leukotriene B4 receptor BLT1 for neutrophil recruitment in inflammatory arthritis
Abstract
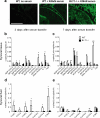
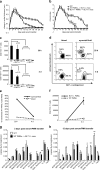
Neutrophil recruitment into tissue plays an important role in host defense and disease pathogenesis, including the inflammatory arthritides. A multitude of diverse chemoattractants have been implicated in neutrophil recruitment, suggesting that they have overlapping functions in mediating this critical biological response. However, here we demonstrate a unique, non-redundant role for the leukotriene B4 receptor BLT1 in mediating neutrophil recruitment into the joint in the K/BxN mouse model of inflammatory arthritis. We demonstrate that neutrophil expression of BLT1 was absolutely required for arthritis generation and chemokine production in this model, and that specific BLT1 inhibition reversed established disease. Adoptive transfer of wild-type (WT) neutrophils restored arthritis and chemokine production in BLT1(-/-) mice. Surprisingly, the primary effect of the transferred WT neutrophils into BLT1(-/-) mice was to promote the entry of endogenous BLT1(-/-) neutrophils into the joints of these mice. However, continued joint inflammation was dependent on the presence of WT neutrophils, indicating an ongoing specific requirement for BLT1-activated neutrophils in mediating BLT1(-/-) neutrophil recruitment by other chemoattractants. These experiments demonstrate that neutrophil BLT1 functions in a novel and essential non-cell-autonomous manner to enable the recruitment of additional neutrophils not expressing this receptor, thereby amplifying the inflammatory response in autoantibody-induced arthritis.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

