CD4+ T cells that enter the draining lymph nodes after antigen injection participate in the primary response and become central-memory cells
- PMID: 16567390
- PMCID: PMC2118291
- DOI: 10.1084/jem.20051954
CD4+ T cells that enter the draining lymph nodes after antigen injection participate in the primary response and become central-memory cells
Abstract
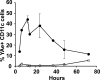
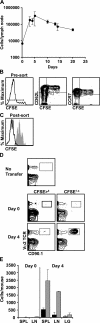
We explored the relationship between the time of naive CD4+ T cell exposure to antigen in the primary immune response and the quality of the memory cells produced. Naive CD4+ T cells that migrated into the skin-draining lymph nodes after subcutaneous antigen injection accounted for about half of the antigen-specific population present at the peak of clonal expansion. These late-arriving T cells divided less and more retained the central-memory marker CD62L than the T cells that resided in the draining lymph nodes at the time of antigen injection. The fewer cell divisions were related to competition with resident T cells that expanded earlier in the response and a reduction in the number of dendritic cells displaying peptide-major histocompatibility complex (MHC) II complexes at later times after antigen injection. The progeny of late-arriving T cells possessed the phenotype of central-memory cells, and proliferated more extensively during the secondary response than the progeny of the resident T cells. The results suggest that late arrival into lymph nodes and exposure to antigen-presenting cells displaying lower numbers of peptide-MHC II complexes in the presence of competing T cells ensures that some antigen-specific CD4+ T cells divide less in the primary response and become central-memory cells.
Figures




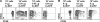
References
-
- Jenkins, M.K., A. Khoruts, E. Ingulli, D.L. Mueller, S.J. McSorley, R.L. Reinhardt, A. Itano, and K.A. Pape. 2001. In vivo activation of antigen-specific CD4 T cells. Annu. Rev. Immunol. 19:23–45. - PubMed
-
- Seder, R.A., and R. Ahmed. 2003. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat. Immunol. 4:835–842. - PubMed
-
- Sallusto, F., D. Lenig, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 401:708–712. - PubMed
-
- Reinhardt, R.L., A. Khoruts, R. Merica, T. Zell, and M.K. Jenkins. 2001. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 410:101–105. - PubMed
-
- Masopust, D., V. Vezys, A.L. Marzo, and L. Lefrancois. 2001. Preferential localization of effector–memory cells in nonlymphoid tissue. Science. 291:2413–2417. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

