Processing of human cathepsin D is independent of its catalytic function and auto-activation: involvement of cathepsins L and B
- PMID: 16567401
- PMCID: PMC2376303
- DOI: 10.1093/jb/mvj037
Processing of human cathepsin D is independent of its catalytic function and auto-activation: involvement of cathepsins L and B
Abstract
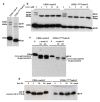
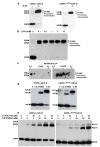
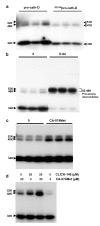
The current mechanism proposed for the processing and activation of the 52 kDa lysosomal aspartic protease cathepsin D (cath-D) is a combination of partial auto-activation generating a 51 kDa pseudo-cath-D, followed by enzyme-assisted maturation involving cysteine and/or aspartic proteases and yielding successively a 48 kDa intermediate and then 34 + 14 kDa cath-D mature species. Here we have investigated the in vivo processing of human cath-D in a cath-D-deficient fibroblast cell line in order to determine whether its maturation occurs through already active cath-D and/or other proteases. We demonstrate that cellular cath-D is processed in a manner independent of its catalytic function and that auto-activation is not a required step. Moreover, the cysteine protease inhibitor E-64 partially blocks processing, leading to accumulation of 52-48 kDa cath-D intermediates. Furthermore, two inhibitors, CLICK148 and CA-074Met, specific for the lysosomal cath-L and cath-B cysteine proteases induce accumulation of 48 kDa intermediate cath-D. Finally, maturation of endocytosed pro-cath-D is also independent of its catalytic function and requires cysteine proteases. We therefore conclude that the mechanism of cath-D maturation involves a fully-assisted processing similar to that of pro-renin.
Figures

References
-
- Tang J, Wong RN. Evolution in the structure and function of aspartic proteases. J Cell Biochem. 1987;33:53–63. - PubMed
-
- Hsueh WA, Baxter JD. Human prorenin. Hypertension. 1991;17:469–477. - PubMed
-
- Richo G, Conner GE. Proteolytic activation of human procathepsin D. Adv Exp Med Biol. 1991;306:289–296. - PubMed
-
- Conner GE, Richo G. Isolation and characterization of a stable activation intermediate of the lysosomal aspartyl protease cathepsin D. Biochem. 1992;31:1142–1147. - PubMed
-
- Larsen LB, Boisen A, Petersen TE. Procathepsin D cannot autoactivate to cathepsin D at acid pH. FEBS Lett. 1993;319:54–58. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

