Mechanism of beta4 subunit modulation of BK channels
- PMID: 16567466
- PMCID: PMC2151511
- DOI: 10.1085/jgp.200509436
Mechanism of beta4 subunit modulation of BK channels
Abstract
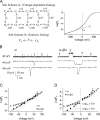
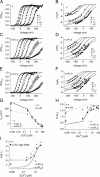
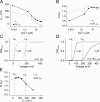
Large-conductance (BK-type) Ca(2+)-activated potassium channels are activated by membrane depolarization and cytoplasmic Ca(2+). BK channels are expressed in a broad variety of cells and have a corresponding diversity in properties. Underlying much of the functional diversity is a family of four tissue-specific accessory subunits (beta1-beta4). Biophysical characterization has shown that the beta4 subunit confers properties of the so-called "type II" BK channel isotypes seen in brain. These properties include slow gating kinetics and resistance to iberiotoxin and charybdotoxin blockade. In addition, the beta4 subunit reduces the apparent voltage sensitivity of channel activation and has complex effects on apparent Ca(2+) sensitivity. Specifically, channel activity at low Ca(2+) is inhibited, while at high Ca(2+), activity is enhanced. The goal of this study is to understand the mechanism underlying beta4 subunit action in the context of a dual allosteric model for BK channel gating. We observed that beta4's most profound effect is a decrease in P(o) (at least 11-fold) in the absence of calcium binding and voltage sensor activation. However, beta4 promotes channel opening by increasing voltage dependence of P(o)-V relations at negative membrane potentials. In the context of the dual allosteric model for BK channels, we find these properties are explained by distinct and opposing actions of beta4 on BK channels. beta4 reduces channel opening by decreasing the intrinsic gating equilibrium (L(0)), and decreasing the allosteric coupling between calcium binding and voltage sensor activation (E). However, beta4 has a compensatory effect on channel opening following depolarization by shifting open channel voltage sensor activation (Vh(o)) to more negative membrane potentials. The consequence is that beta4 causes a net positive shift of the G-V relationship (relative to alpha subunit alone) at low calcium. At higher calcium, the contribution by Vh(o) and an increase in allosteric coupling to Ca(2+) binding (C) promotes a negative G-V shift of alpha+beta4 channels as compared to alpha subunits alone. This manner of modulation predicts that type II BK channels are downregulated by beta4 at resting voltages through effects on L(0). However, beta4 confers a compensatory effect on voltage sensor activation that increases channel opening during depolarization.
Figures








Similar articles
-
An S6 mutation in BK channels reveals beta1 subunit effects on intrinsic and voltage-dependent gating.J Gen Physiol. 2006 Dec;128(6):731-44. doi: 10.1085/jgp.200609596. J Gen Physiol. 2006. PMID: 17130522 Free PMC article.
-
Coupling between voltage sensor activation, Ca2+ binding and channel opening in large conductance (BK) potassium channels.J Gen Physiol. 2002 Sep;120(3):267-305. doi: 10.1085/jgp.20028605. J Gen Physiol. 2002. PMID: 12198087 Free PMC article.
-
Regulation of STREX exon large conductance, calcium-activated potassium channels by the beta4 accessory subunit.Neuroscience. 2007 Nov 23;149(4):789-803. doi: 10.1016/j.neuroscience.2007.07.066. Epub 2007 Sep 12. Neuroscience. 2007. PMID: 17945424 Free PMC article.
-
Current understanding of iberiotoxin-resistant BK channels in the nervous system.Front Physiol. 2014 Oct 9;5:382. doi: 10.3389/fphys.2014.00382. eCollection 2014. Front Physiol. 2014. PMID: 25346692 Free PMC article. Review.
-
Modulation of BK Channel Function by Auxiliary Beta and Gamma Subunits.Int Rev Neurobiol. 2016;128:51-90. doi: 10.1016/bs.irn.2016.03.015. Epub 2016 Apr 8. Int Rev Neurobiol. 2016. PMID: 27238261 Free PMC article. Review.
Cited by
-
Molecular mechanism underlying β1 regulation in voltage- and calcium-activated potassium (BK) channels.Proc Natl Acad Sci U S A. 2015 Apr 14;112(15):4809-14. doi: 10.1073/pnas.1504378112. Epub 2015 Mar 30. Proc Natl Acad Sci U S A. 2015. PMID: 25825713 Free PMC article.
-
Modulation of BK channel voltage gating by different auxiliary β subunits.Proc Natl Acad Sci U S A. 2012 Nov 13;109(46):18991-6. doi: 10.1073/pnas.1216953109. Epub 2012 Oct 29. Proc Natl Acad Sci U S A. 2012. PMID: 23112204 Free PMC article.
-
Challenges in the Therapeutic Targeting of KCa Channels: From Basic Physiology to Clinical Applications.Int J Mol Sci. 2024 Mar 4;25(5):2965. doi: 10.3390/ijms25052965. Int J Mol Sci. 2024. PMID: 38474212 Free PMC article. Review.
-
An unexpected journey: conceptual evolution of mechanoregulated potassium transport in the distal nephron.Am J Physiol Cell Physiol. 2016 Feb 15;310(4):C243-59. doi: 10.1152/ajpcell.00328.2015. Epub 2015 Dec 2. Am J Physiol Cell Physiol. 2016. PMID: 26632600 Free PMC article. Review.
-
Calcium-Activated K+ Channels (KCa) and Therapeutic Implications.Handb Exp Pharmacol. 2021;267:379-416. doi: 10.1007/164_2021_459. Handb Exp Pharmacol. 2021. PMID: 33945030
References
-
- Behrens, R., A. Nolting, F. Reimann, M. Schwarz, R. Waldschutz, and O. Pongs. 2000. hKCNMB3 and hKCNMB4, cloning and characterization of two members of the large-conductance calcium-activated potassium channel β subunit family. FEBS Lett. 474:99–106. - PubMed
-
- Brenner, R., Q.H. Chen, A. Vilaythong, G.M. Toney, J.L. Noebels, and R.W. Aldrich. 2005. BK channel β4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat. Neurosci. 8:1752–1759. - PubMed
-
- Brenner, R., T.J. Jegla, A. Wickenden, Y. Liu, and R.W. Aldrich. 2000. Cloning and functional characterization of novel large conductance calcium-activated potassium channel β subunits, hKCNMB3 and hKCNMB4. J. Biol. Chem. 275:6453–6461. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

