Stu2p binds tubulin and undergoes an open-to-closed conformational change
- PMID: 16567500
- PMCID: PMC2063759
- DOI: 10.1083/jcb.200511010
Stu2p binds tubulin and undergoes an open-to-closed conformational change
Abstract
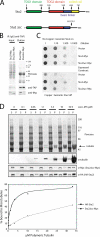
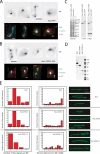
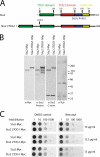
Stu2p from budding yeast belongs to the conserved Dis1/XMAP215 family of microtubule-associated proteins (MAPs). The common feature of proteins in this family is the presence of HEAT repeat-containing TOG domains near the NH2 terminus. We have investigated the functions of the two TOG domains of Stu2p in vivo and in vitro. Our data suggest that Stu2p regulates microtubule dynamics through two separate activities. First, Stu2p binds to a single free tubulin heterodimer through its first TOG domain. A large conformational transition in homodimeric Stu2p from an open structure to a closed one accompanies the capture of a single free tubulin heterodimer. Second, Stu2p has the capacity to associate directly with microtubule ends, at least in part, through its second TOG domain. These two properties lead to the stabilization of microtubules in vivo, perhaps by the loading of tubulin dimers at microtubule ends. We suggest that this mechanism of microtubule regulation is a conserved feature of the Dis1/XMAP215 family of MAPs.
Figures




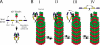
References
-
- Carvalho, P., J.S. Tirnauer, and D. Pellman. 2003. Surfing on microtubule ends. Trends Cell Biol. 13:229–237. - PubMed
-
- Cassimeris, L., D. Gard, P.T. Tran, and H.P. Erickson. 2001. XMAP215 is a long thin molecule that does not increase microtubule stiffness. J. Cell Sci. 114:3025–3033. - PubMed
-
- Charrasse, S., M. Schroeder, C. Gauthier-Rouviere, F. Ango, L. Cassimeris, D.L. Gard, and C. Larroque. 1998. The TOGp protein is a new human microtubule-associated protein homologous to the Xenopus XMAP215. J. Cell Sci. 111:1371–1383. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

