Vectorial insertion of apical and basolateral membrane proteins in polarized epithelial cells revealed by quantitative 3D live cell imaging
- PMID: 16567501
- PMCID: PMC2063761
- DOI: 10.1083/jcb.200512012
Vectorial insertion of apical and basolateral membrane proteins in polarized epithelial cells revealed by quantitative 3D live cell imaging
Abstract
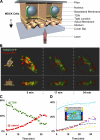
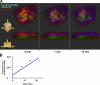
Although epithelial cells are known to exhibit a polarized distribution of membrane components, the pathways responsible for delivering membrane proteins to their appropriate domains remain unclear. Using an optimized approach to three-dimensional live cell imaging, we have visualized the transport of newly synthesized apical and basolateral membrane proteins in fully polarized filter-grown Madin-Darby canine kidney cells. We performed a detailed quantitative kinetic analysis of trans-Golgi network (TGN) exit, passage through transport intermediates, and arrival at the plasma membrane using cyan/yellow fluorescent protein-tagged glycosylphosphatidylinositol-anchored protein and vesicular stomatitis virus glycoprotein as apical and basolateral reporters, respectively. For both pathways, exit from the TGN was rate limiting. Furthermore, apical and basolateral proteins were targeted directly to their respective membranes, resolving current confusion as to whether sorting occurs on the secretory pathway or only after endocytosis. However, a transcytotic protein did reach the apical surface after a prior appearance basolaterally. Finally, newly synthesized proteins appeared to be delivered to the entire lateral or apical surface, suggesting-contrary to expectations-that there is not a restricted site for vesicle docking or fusion adjacent to the junctional complex.
Figures



Comment in
-
Controversy fuels trafficking of GPI-anchored proteins.J Cell Biol. 2006 Mar 27;172(7):963-5. doi: 10.1083/jcb.200603015. J Cell Biol. 2006. PMID: 16567497 Free PMC article.
References
-
- Brown, P.S., E. Wang, B. Aroeti, S.J. Chapin, K.E. Mostov, and K.W. Dunn. 2000. Definition of distinct compartments in polarized Madin-Darby canine kidney (MDCK) cells for membrane-volume sorting, polarized sorting and apical recycling. Traffic. 1:124–140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

